Question: Can you please provide the code using matlab to get the 2 plots needed. simulator and is ready to be used. 1) Validation of your

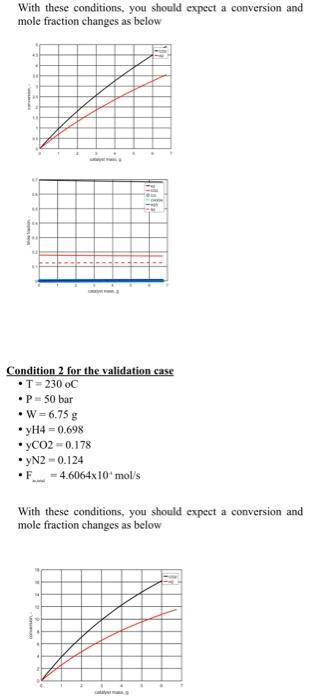
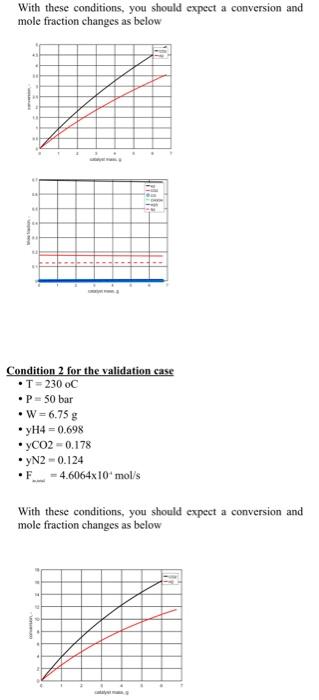
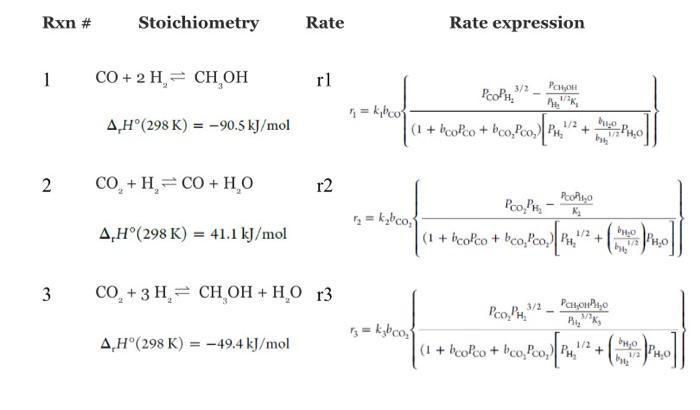


simulator and is ready to be used. 1) Validation of your kinetic model: Set up the reactor model equations in a numerical solver and validate your code versus the experimental conditions provided in the next page. 2) Initial assessment on how to choose optimal conditions: If you have the chance to add or remove catalyst from this reactor, how much you will add or remove considering the ultimate purpose of why we are interested in this reaction? This part you can answer based on the results of part one. No need to do any simulation or calculations. You need to make a plot of how the mole fractions are changing along the reactor bed. Main learning outcome- start to learn how to choose optimal conditions for an application of multiple reactions and components and think of options. 3) Base case conditions: Once your simulation code is validated, use it to find out the required catalyst amount required to reach a CO2 conversion of 30% for a temperature of 230C, a pressure of 50 bar, and a hydrogen mole fraction of 0.698,CO2 mole fraction of 0.178 , and remaining inert (you can assume the inert is nitrogen), and for a total inlet molar flow rate of 4.6064104mol/sec. Also, calculate what will be the space-time in this case. Main learning outcome- Sizing an industrial reactor at base case conditions which will be used as a reference point to do a parametric study. Condition 1 for the validation case - T=200oC - P=50bar - W=6.75g - yH4=0.698 - yCO2=0.178 yN2=0.124 - F=4.6064104mol/s With these conditions, you should expect a conversion and mole fraction changes as below Condition 2 for the validation case - T=230oC - P=50 bar - W=6.75g - yH4=0.698 - yCO2=0.178 - yN2=0.124 - F2=4.6064104mol/s With these conditions, you should expect a conversion and mole fraction changes as below With these conditions, you should expect a conversion and mole fraction changes as below Condition 2 for the validation case - T=230oC - P=50 bar - W=6.75g - yH4=0.698 - yCO2=0.178 - yN2=0.124 - F2=4.6064104mol/s With these conditions, you should expect a conversion and mole fraction changes as below Stoichiometry Rate Rate expression Supporting informations to help you in preparing the matlab code To ensure you don't have any mistakes while copying the reaction rate expression, I am providing you with a code that was used with the given kinetics in matlab. Important note: If you want to use this code, you need to make sure you are using the same variable names as Matlab is case-sensitive. Define Adsorption constants: bCO= 2.1610(5)exp(46800/(RcppT)); bCO2= 7.0510(7))exp(61700/(RCPp.T)); bfrac= 6.3710(9)exp(84000/(RCPPT)); efine Equilibrium Constants: K1=10((5139/T)12.621); K2=10((2073/T)+2.029); K3=10((3066/T)10.592); 88. Base Rate Expressions r1=k1bCO((P3P1(3/2) P4/(P1(1/2)K1))/((1+bCOP3+bCO2P2) (P1(1/2)+bfracP5))); r2=k2bCO2((P2P1 P3P5/K2)/((1+bCOP3+bCO2P2) (P1(1/2)+bfraC5))); r3=k3bCO2((P2P1(3/2) P4P5/(P1(3/2)K3))/((1+bCOP3+bCO2P 2)(P1(1/2)+bfracP5))); simulator and is ready to be used. 1) Validation of your kinetic model: Set up the reactor model equations in a numerical solver and validate your code versus the experimental conditions provided in the next page. 2) Initial assessment on how to choose optimal conditions: If you have the chance to add or remove catalyst from this reactor, how much you will add or remove considering the ultimate purpose of why we are interested in this reaction? This part you can answer based on the results of part one. No need to do any simulation or calculations. You need to make a plot of how the mole fractions are changing along the reactor bed. Main learning outcome- start to learn how to choose optimal conditions for an application of multiple reactions and components and think of options. 3) Base case conditions: Once your simulation code is validated, use it to find out the required catalyst amount required to reach a CO2 conversion of 30% for a temperature of 230C, a pressure of 50 bar, and a hydrogen mole fraction of 0.698,CO2 mole fraction of 0.178 , and remaining inert (you can assume the inert is nitrogen), and for a total inlet molar flow rate of 4.6064104mol/sec. Also, calculate what will be the space-time in this case. Main learning outcome- Sizing an industrial reactor at base case conditions which will be used as a reference point to do a parametric study. Condition 1 for the validation case - T=200oC - P=50bar - W=6.75g - yH4=0.698 - yCO2=0.178 yN2=0.124 - F=4.6064104mol/s With these conditions, you should expect a conversion and mole fraction changes as below Condition 2 for the validation case - T=230oC - P=50 bar - W=6.75g - yH4=0.698 - yCO2=0.178 - yN2=0.124 - F2=4.6064104mol/s With these conditions, you should expect a conversion and mole fraction changes as below With these conditions, you should expect a conversion and mole fraction changes as below Condition 2 for the validation case - T=230oC - P=50 bar - W=6.75g - yH4=0.698 - yCO2=0.178 - yN2=0.124 - F2=4.6064104mol/s With these conditions, you should expect a conversion and mole fraction changes as below Stoichiometry Rate Rate expression Supporting informations to help you in preparing the matlab code To ensure you don't have any mistakes while copying the reaction rate expression, I am providing you with a code that was used with the given kinetics in matlab. Important note: If you want to use this code, you need to make sure you are using the same variable names as Matlab is case-sensitive. Define Adsorption constants: bCO= 2.1610(5)exp(46800/(RcppT)); bCO2= 7.0510(7))exp(61700/(RCPp.T)); bfrac= 6.3710(9)exp(84000/(RCPPT)); efine Equilibrium Constants: K1=10((5139/T)12.621); K2=10((2073/T)+2.029); K3=10((3066/T)10.592); 88. Base Rate Expressions r1=k1bCO((P3P1(3/2) P4/(P1(1/2)K1))/((1+bCOP3+bCO2P2) (P1(1/2)+bfracP5))); r2=k2bCO2((P2P1 P3P5/K2)/((1+bCOP3+bCO2P2) (P1(1/2)+bfraC5))); r3=k3bCO2((P2P1(3/2) P4P5/(P1(3/2)K3))/((1+bCOP3+bCO2P 2)(P1(1/2)+bfracP5)))
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


