Question: can you please type it too if its possible? or if it's on paper can you scan it or something with good quality please thanks
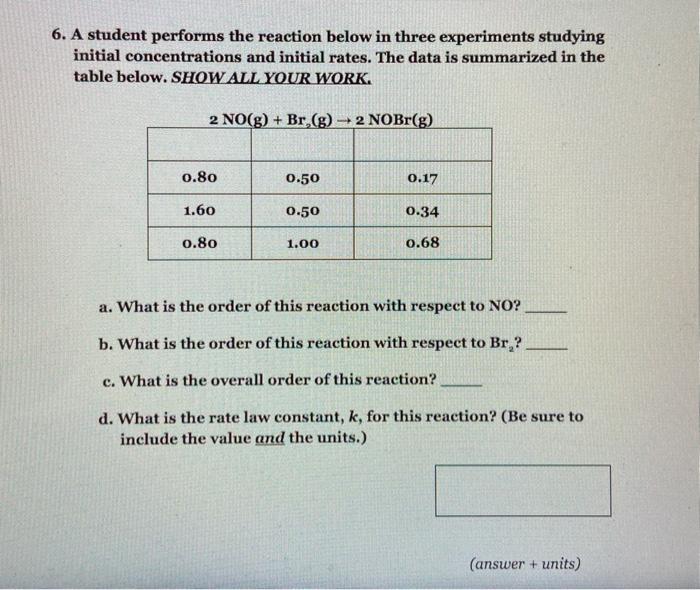
6. A student performs the reaction below in three experiments studying initial concentrations and initial rates. The data is summarized in the table below. SHOW ALL YOUR WORK. 2 NO(g) + Br,(g) +2 NOBr(g) 0.80 0.50 0.17 1.60 0.50 0.34 0.80 1.00 0.68 a. What is the order of this reaction with respect to NO? b. What is the order of this reaction with respect to Br? c. What is the overall order of this reaction? d. What is the rate law constant, k, for this reaction? (Be sure to include the value and the units.) (answer + units)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


