Question: can you solve that pls Question 3(0.5) : The average rate of formation of NO2 over a certain period, for the decomposition reaction 2N2O5(g)4NO2(g)+O2(g) is
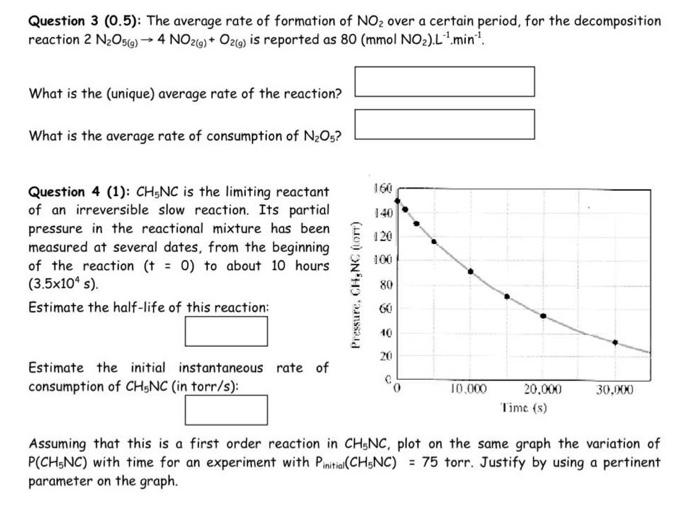
Question 3(0.5) : The average rate of formation of NO2 over a certain period, for the decomposition reaction 2N2O5(g)4NO2(g)+O2(g) is reported as 80(mmolNO2)L1min1. What is the (unique) average rate of the reaction? What is the average rate of consumption of N2O5 ? Question 4 (1): CH5NC is the limiting reactant of an irreversible slow reaction. Its partial pressure in the reactional mixture has been measured at several dates, from the beginning of the reaction (t=0) to about 10 hours (3.5104s). Estimate the half-life of this reaction: Estimate the initial instantaneous rate of consumption of CH5NC (in torr/s): Assuming that this is a first order reaction in CH5NC, plot on the same graph the variation of P(CH5NC) with time for an experiment with Pinitial(CH5NC)=75 torr. Justify by using a pertinent parameter on the graph
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


