Question: can you telk me the steps to solve this problem regarding resonance For which of the following compounds does bonding / resonance produce a lowest
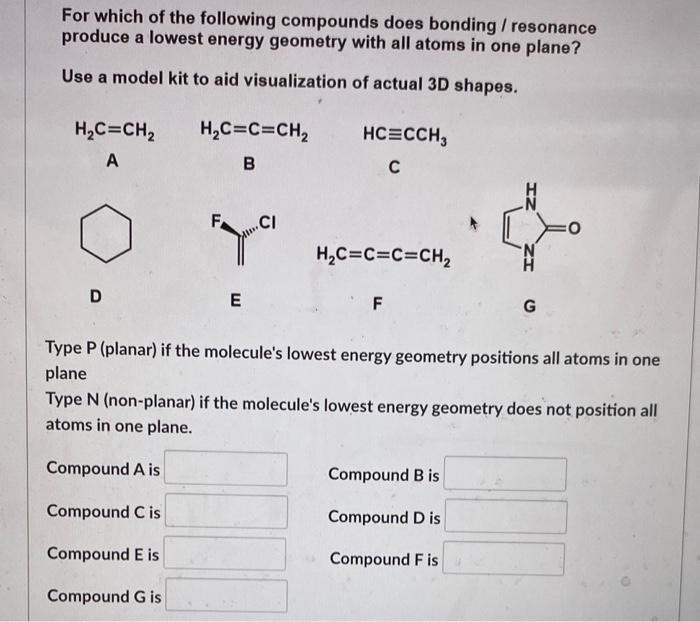
For which of the following compounds does bonding / resonance produce a lowest energy geometry with all atoms in one plane? Use a model kit to aid visualization of actual 3D shapes. Type P (planar) if the molecule's lowest energy geometry positions all atoms in one plane Type N (non-planar) if the molecule's lowest energy geometry does not position all atoms in one plane. Compound A is Compound B is Compound C is Compound D is Compound E is Compound F is Compound G is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


