Question: CaO can be used as a drying agent. One such application occurs when water is added to dry concrete or cement. The reaction that
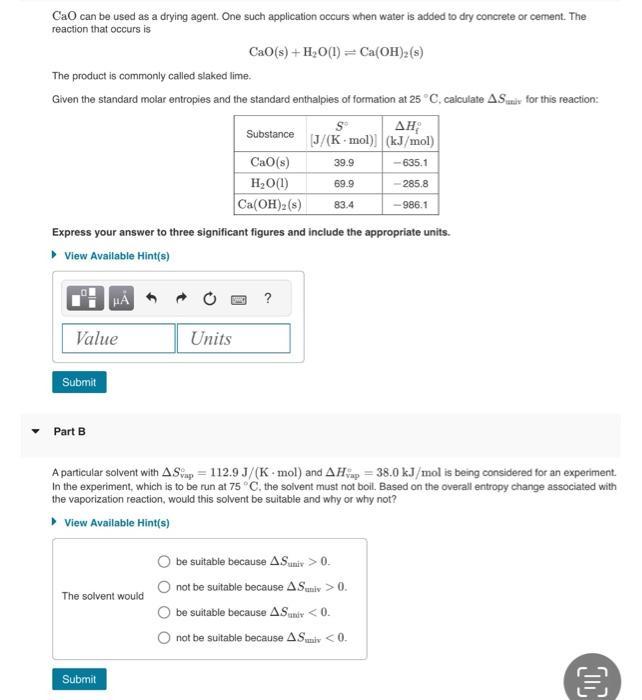
CaO can be used as a drying agent. One such application occurs when water is added to dry concrete or cement. The reaction that occurs is Cao(s) + H2O(1)=Ca(OH)2(s) The product is commonly called slaked lime. Given the standard molar entropies and the standard enthalpies of formation at 25 C, calculate AS for this reaction: Substance [J/(K mol)] (kJ/mol) CaO(s) 39.9 -635.1 HO(1) Ca(OH)2(s) 69.9 -285.8 83.4 -986.1 Express your answer to three significant figures and include the appropriate units. View Available Hint(s) ? Value Units Submit Part B A particular solvent with AS ap 112.9 J/(K mol) and AH 38.0 kJ/mol is being considered for an experiment. In the experiment, which is to be run at 75 C. the solvent must not boil. Based on the overall entropy change associated with the vaporization reaction, would this solvent be suitable and why or why not? View Available Hint(s) The solvent would be suitable because A.Suniv > 0. not be suitable because A.Suniv > 0. be suitable because AS univ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


