Question: CARBON DIOXIDE ( left ( mathrm { CO } _ { 2 } right ) ) and NITROGEN (
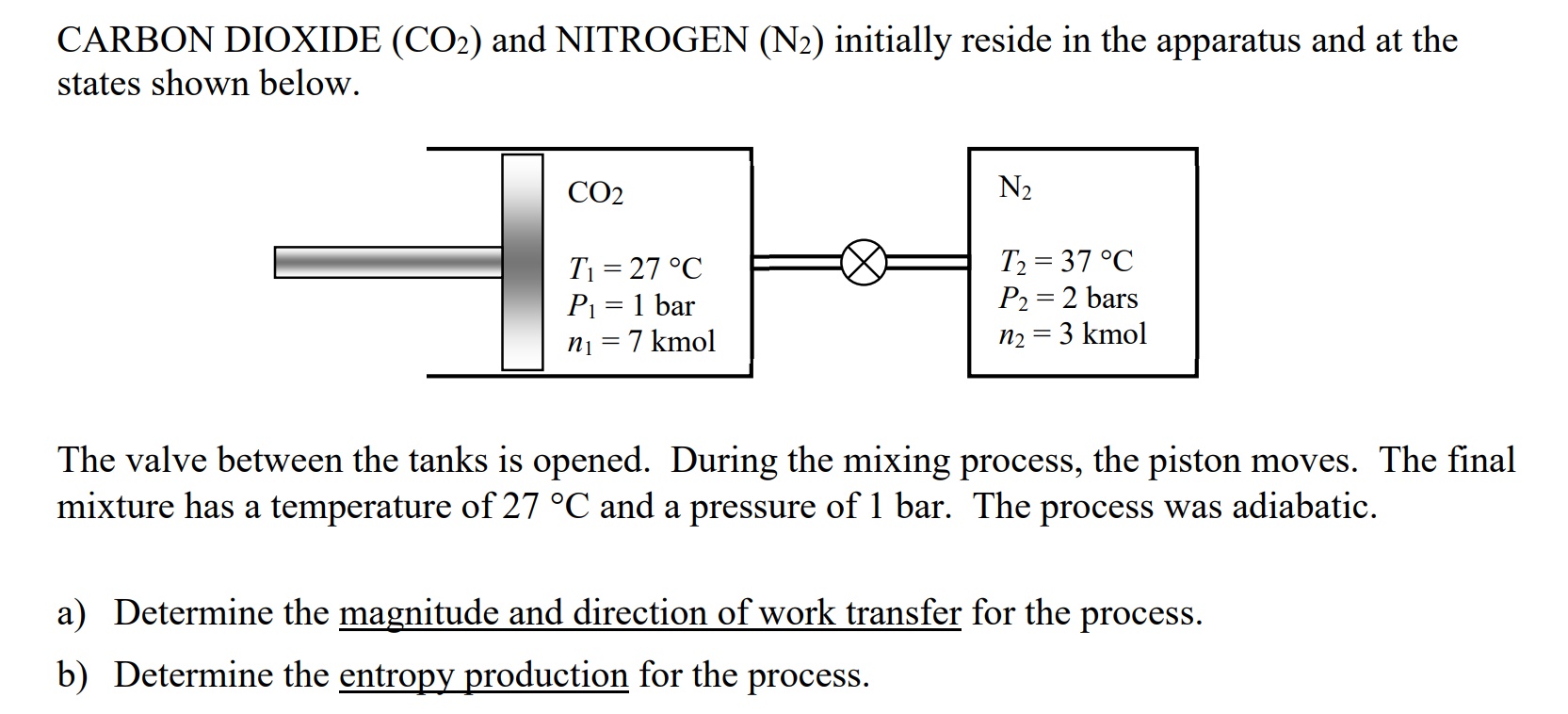
CARBON DIOXIDE leftmathrmCOright and NITROGEN leftmathrmNright initially reside in the apparatus and at the states shown below.
The valve between the tanks is opened. During the mixing process, the piston moves. The final mixture has a temperature of circmathrmC and a pressure of bar. The process was adiabatic.
a Determine the magnitude and direction of work transfer for the process.
b Determine the entropy production for the process.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


