Question: cb 816.011Q At what temperature will nitrogen (N2) dissociate to form 2% monatomic nitrogen (N) if the pressure is a) 1kPa and b) 10kPa ?
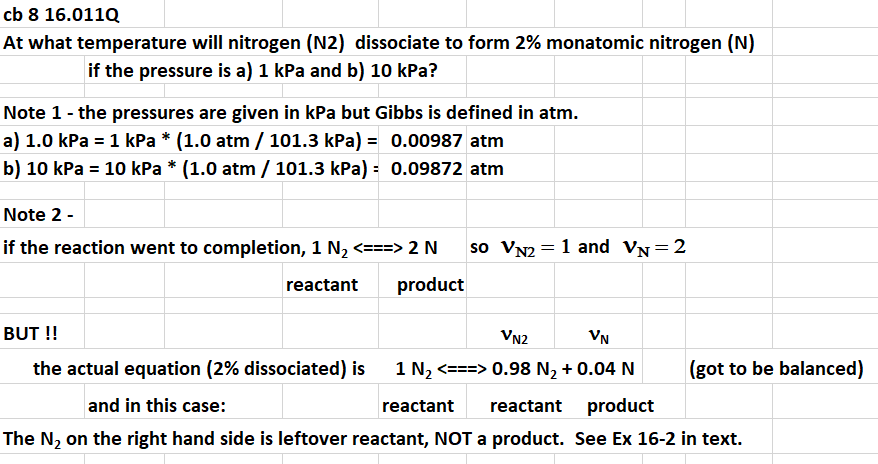
cb 816.011Q At what temperature will nitrogen (N2) dissociate to form 2% monatomic nitrogen (N) if the pressure is a) 1kPa and b) 10kPa ? Note 1 - the pressures are given in kPa but Gibbs is defined in atm. a) 1.0kPa=1kPa(1.0atm/101.3kPa)=0.00987atm b) 10kPa=10kPa(1.0atm/101.3kPa)=0.09872atm Note 2 - if the reaction went to completion, 1N2==2N so vN2=1 and vN=2 reactant product BUT !! vN2vN the actual equation ( 2% dissociated) is 1N2==>.98N2+0.04N (got to be balanced) and in this case: reactant reactant product The N2 on the right hand side is leftover reactant, NOT a product. See Ex 16-2 in text
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


