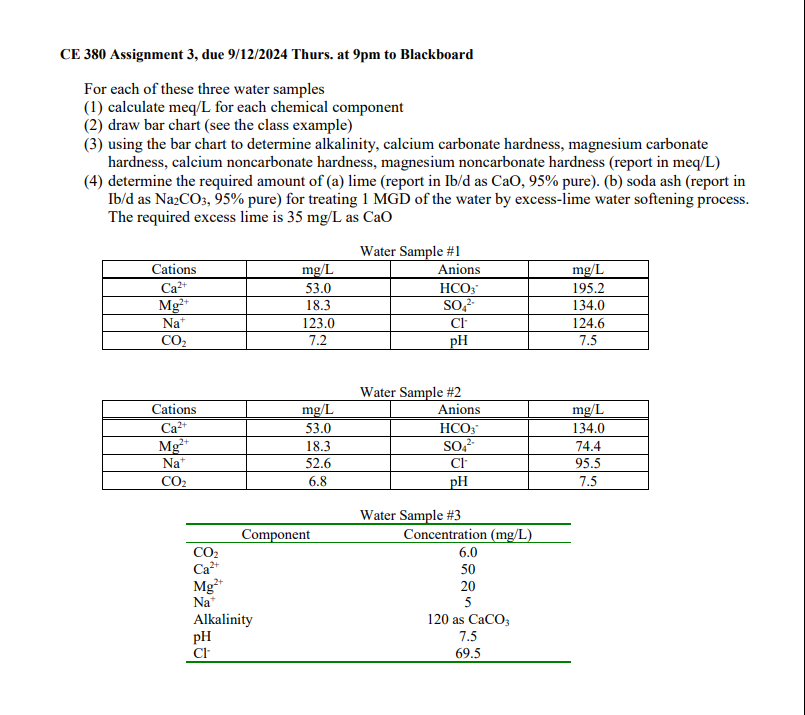
Question: CE 3 8 0 Assignment 3 , due 9 / 1 2 / 2 0 2 4 Thurs. at 9 pm to Blackboard For each
CE Assignment due Thurs. at pm to Blackboard
For each of these three water samples
calculate meqL for each chemical component
draw bar chart see the class example
using the bar chart to determine alkalinity, calcium carbonate hardness, magnesium carbonate
hardness, calcium noncarbonate hardness, magnesium noncarbonate hardness report in meqL
determine the required amount of a lime report in as CaO, pureb soda ash report in
as pure for treating MGD of the water by excesslime water softening process.
The required excess lime is as CaO
Water Sample #
Water Sample #
Water Sample #
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


