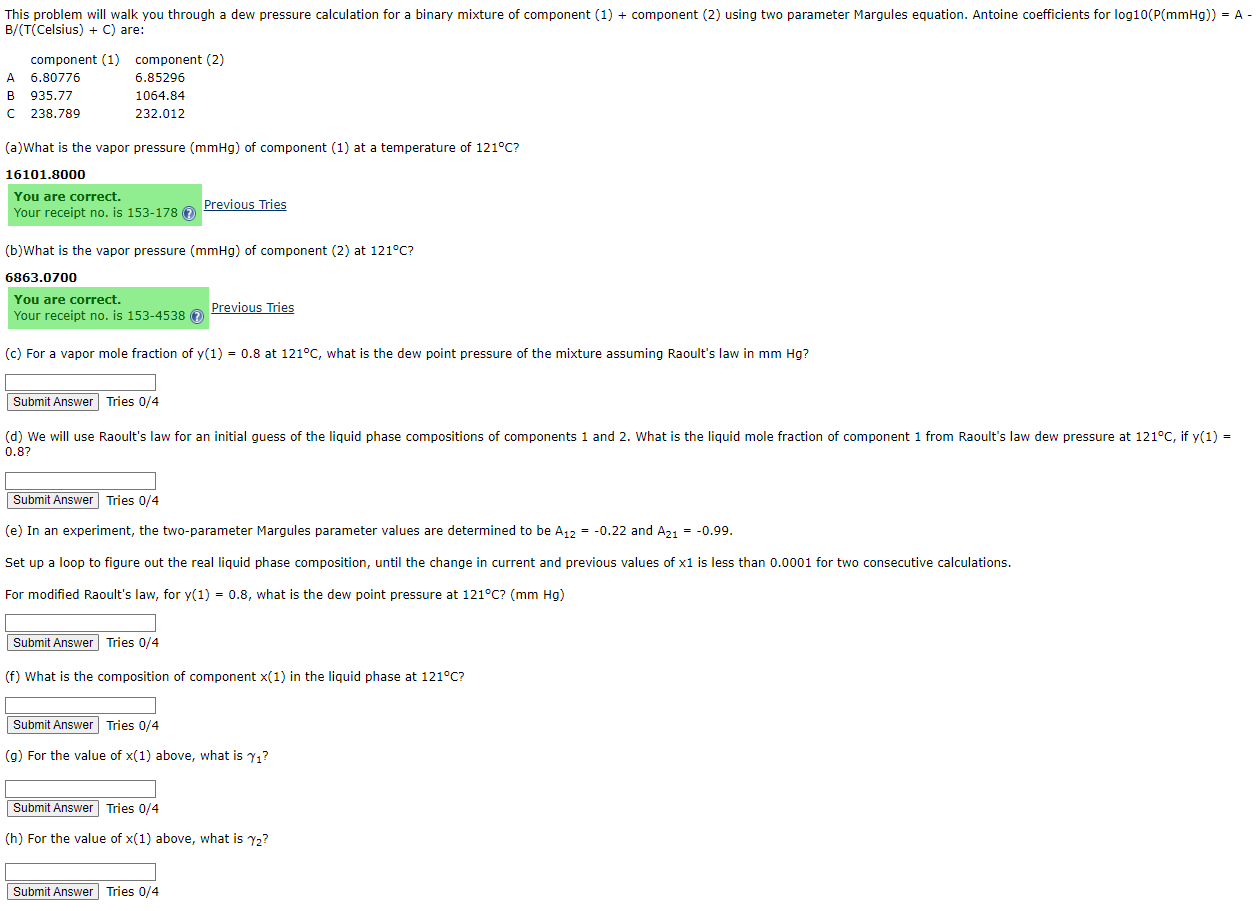
Question: ( Celsius ) + C are: ( a ) What is the vapor pressure ) of component ( 1 ) at a temperature of 1
Celsius are:
aWhat is the vapor pressure of component at a temperature of
You are correct.
Your receipt no is Previous Tries
b What is the vapor pressure of component at
You are correct.
Your receipt no is Previous Tries
c For a vapor mole fraction of at what is the dew point pressure of the mixture assuming Raoult's law in
Tries
Submit Answer Tries
e In an experiment, the twoparameter Margules parameter values are determined to be and
Set up a loop to figure out the real liquid phase composition, until the change in current and previous values of is less than for two consecutive calculations.
For modified Raoult's law, for what is the dew point pressure at
Tries
f What is the composition of component in the liquid phase at
Submit Answer Tries
g For the value of above, what is
Tries
h For the value of above, what is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


