Question: CES PRINTER VERSION BACK NET Chapter 1. Question 63 Parameterization Approximately 12 billion kilograms of phosphoric acid (HPO4) are produced annually for fertilizers, detergents, and
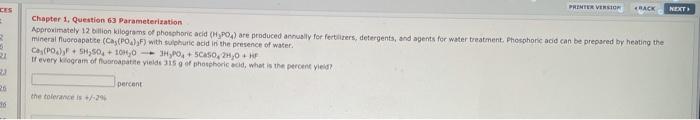
CES PRINTER VERSION BACK NET Chapter 1. Question 63 Parameterization Approximately 12 billion kilograms of phosphoric acid (HPO4) are produced annually for fertilizers, detergents, and agents for water treatment. Phosphoric and can be prepared by heating the mineral Purapatite (Ca, (PO)>F) with sulphuric acid in the presence of water. Ca (Po)FSH,50 + 10,0 ---OSCASO, 2H,0 If every kilogram of fortite yielde is of hosphoric acid, what is the percent yield? I percent 26 the tolerance is +/-29 16
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


