Question: Cesium Chloride ( CsCl ) has a crystal structure that contains C s + ions in the corner of the cube and a C l
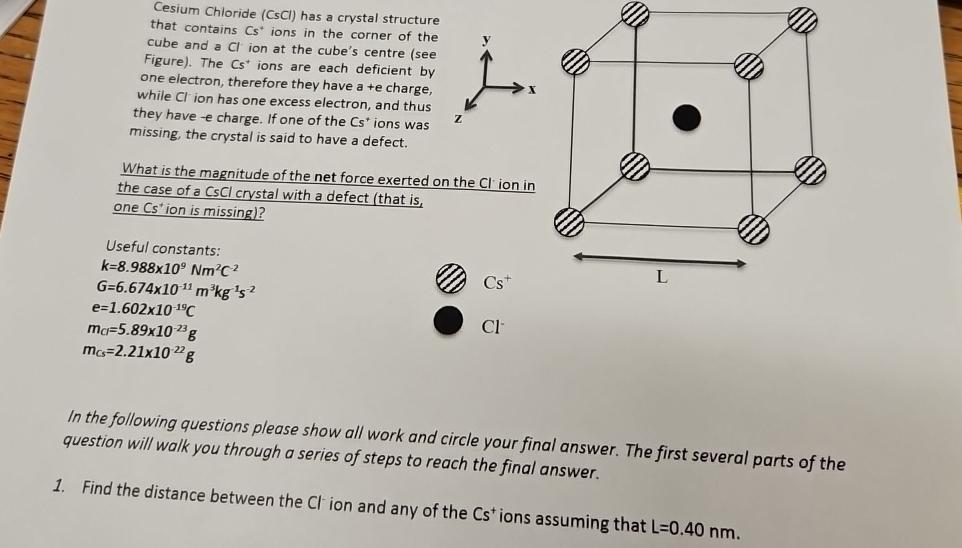
Cesium Chloride CsCl has a crystal structure that contains ions in the corner of the cube and a ion at the cube's centre see Figure The ions are each deficient by one electron, therefore they have a e charge, while ion has one excess electron, and thus they have e charge. If one of the ions was missing, the crystal is said to have a defect.
What is the magnitude of the net force exerted on the ic the case of a CsCl crystal with a defect that is one ion is missing
Useful constants:
In the following questions please show all work and circle your final answer. The first several parts of the question will walk you through a series of steps to reach the final answer.
Find the distance between the ion and any of the ions assuming that
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


