Question: (Ch. 3.9) Given the balanced equation below, identify the limiting reactant when 0.251mol of hydrogen react with 0.182mol of oxygen. 2H2(g)+O2(g)2H2O(l) oxygen The limiting reactant
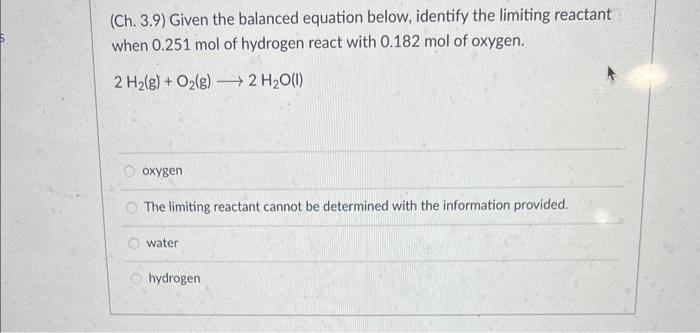
(Ch. 3.9) Given the balanced equation below, identify the limiting reactant when 0.251mol of hydrogen react with 0.182mol of oxygen. 2H2(g)+O2(g)2H2O(l) oxygen The limiting reactant cannot be determined with the information provided. water hydrogen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


