Question: CH 4 Problem Set Question 2 of 2 0 ( 0 . 5 points ) I Question Altempt: 1 of Unlimited 1 2 3 5
CH Problem Set
Question of points I Question Altempt: of Unlimited
Part of
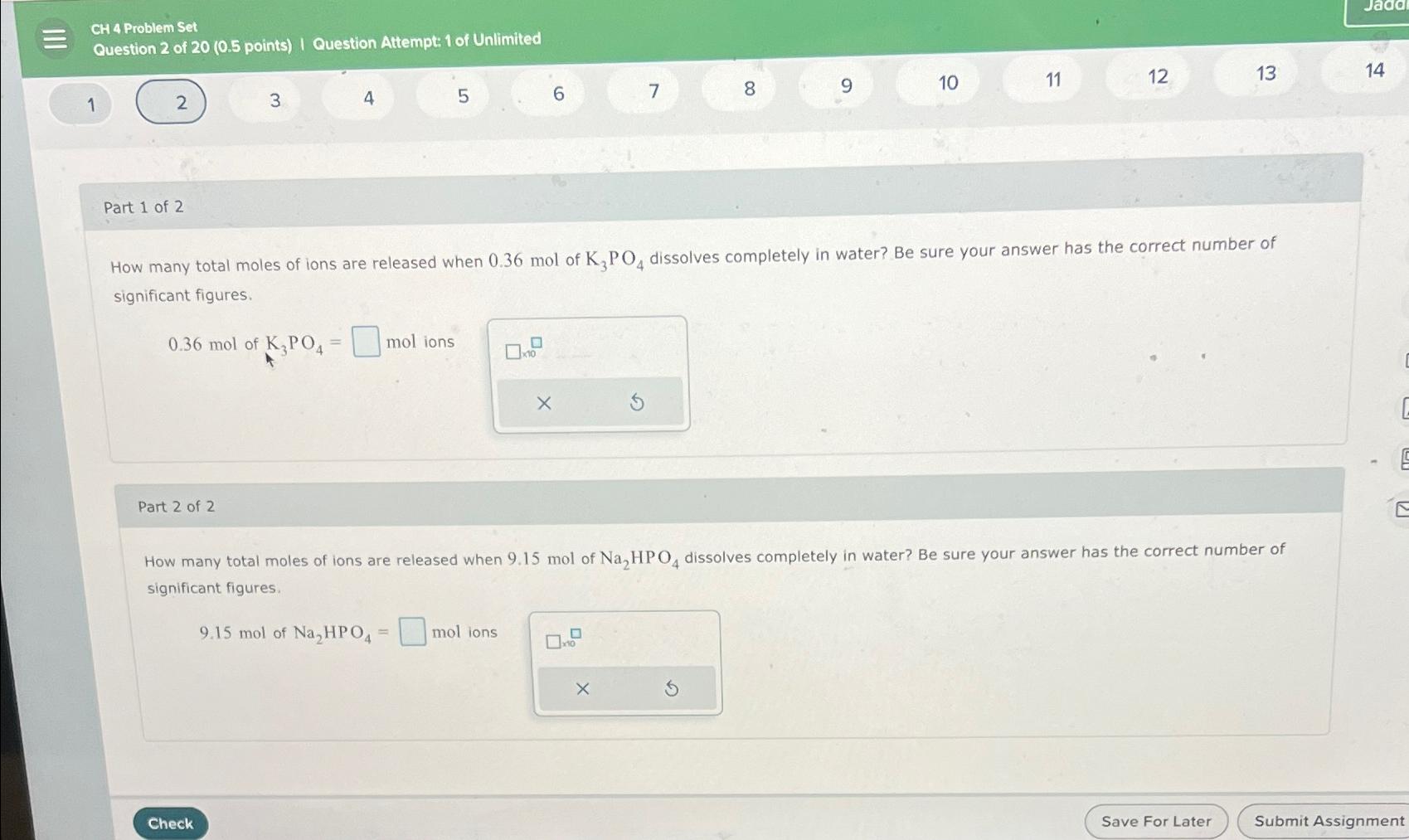
How many total moles of ions are released when mol of dissolves completely in water? Be sure your answer has the correct number of significant figures.
mol mol ions
Part of
How many total moles of ions are released when mol of dissolves completely in water? Be sure your answer has the correct number of significant figures.
mol mol ions
Submit Assignment
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


