Question: Ch. Ex. 26a - lons in Solution 20 10 points Be sure to answer all parts. How many moles and numbers of ions of each
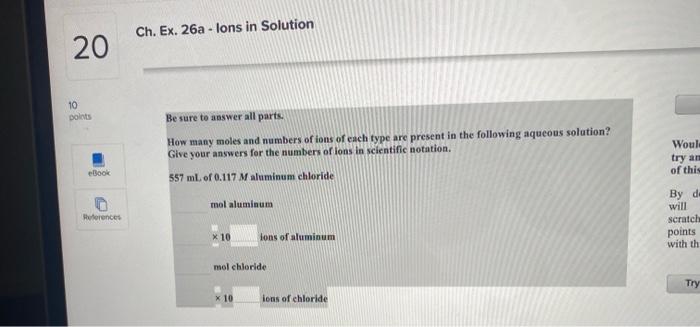
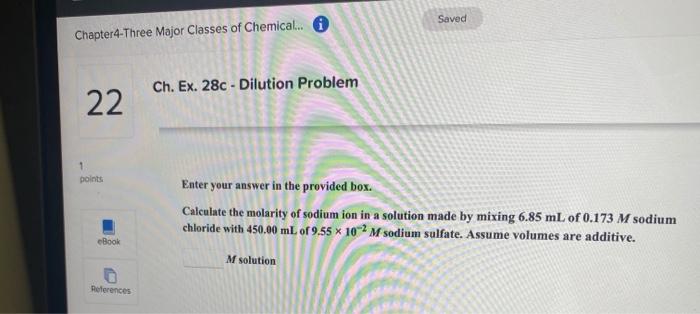
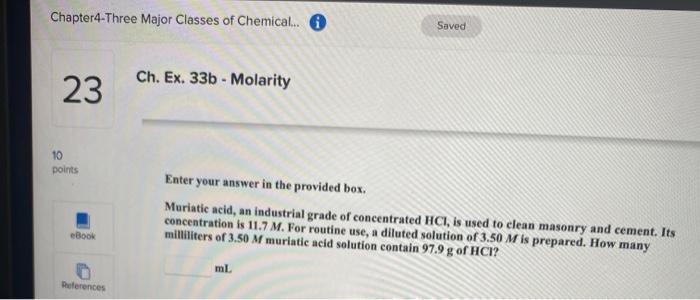
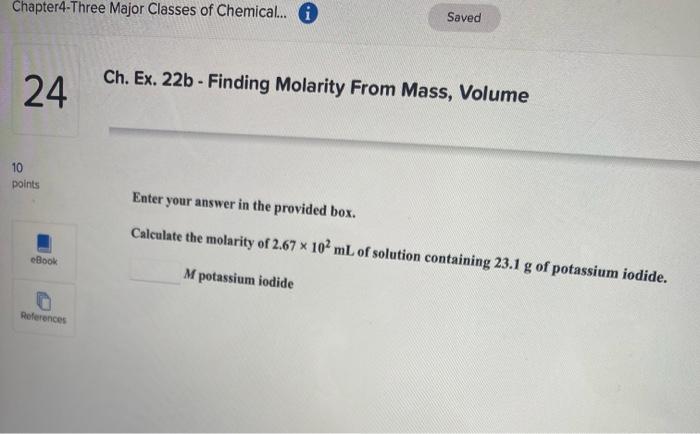
Ch. Ex. 26a - lons in Solution 20 10 points Be sure to answer all parts. How many moles and numbers of ions of each type are present in the following aqueous solution? Give your answers for the numbers of ions in scientific notation. Woul: try an of this eBook 557 ml of 0.117 M aluminum chloride mol aluminum References By do will Scratch points with th x 10 lons of aluminum mol chloride Try x 10 ions of chloride Saved Chapter4-Three Major Classes of Chemical. O Ch. Ex. 28c - Dilution Problem 22 1 points Enter your answer in the provided box. Calculate the molarity of sodium ion in a solution made by mixing 6.85 mL of 0.173 M sodium chloride with 450.00 mL of 9.55 * 10-2 Msodium sulfate. Assume volumes are additive. eBook M solution References Chapter4-Three Major Classes of Chemical... 6 Saved Ch. Ex. 33b - Molarity 23 10 points Enter your answer in the provided box. Muriatic acid, an industrial grade of concentrated HCI, is used to clean masonry and cement. Its concentration is 11.7 M. For routine use, a diluted solution of 3.50 M is prepared. How many milliliters of 3.50 M muriatic acid solution contain 97.9 g of HCI? eBook ml References Chapter4-Three Major Classes of Chemical. O Saved 24 Ch. Ex. 22b - Finding Molarity From Mass, Volume 10 points Enter your answer in the provided box. Calculate the molarity of 2.67 * 10 mL of solution containing 23.1 g of potassium iodide. eBook M potassium iodide References
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


