Question: CH4(g)+H2O(g)CO(g)+3H2(g) Complete the table below, so that it lists the initial molanty of each compound, the change in molarity of each compound due to the
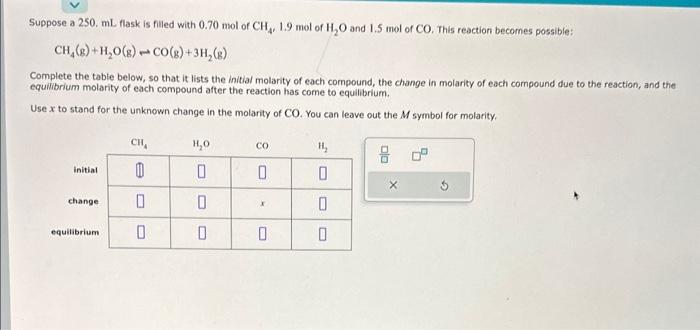
CH4(g)+H2O(g)CO(g)+3H2(g) Complete the table below, so that it lists the initial molanty of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CO. You can leave out the M symbol for molarity
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


