Question: CH4+H2OCO2+3H2 If the equilibrium constant is 1 at a given temperature, what would be the equilibrium conversion for a 1:1 ratio of methane to water
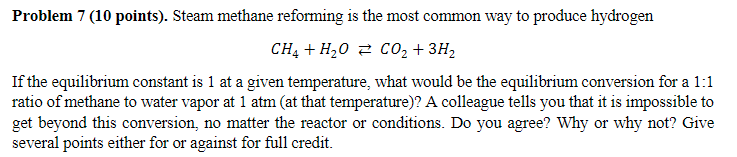
CH4+H2OCO2+3H2 If the equilibrium constant is 1 at a given temperature, what would be the equilibrium conversion for a 1:1 ratio of methane to water vapor at 1atm (at that temperature)? A colleague tells you that it is impossible to get beyond this conversion, no matter the reactor or conditions. Do you agree? Why or why not? Give several points either for or against for full credit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


