Question: Chain rule application problem. The pressure P {in kilopascals), the volume V (in liters), and the temperature T (in kelvins), of one mole of ideal
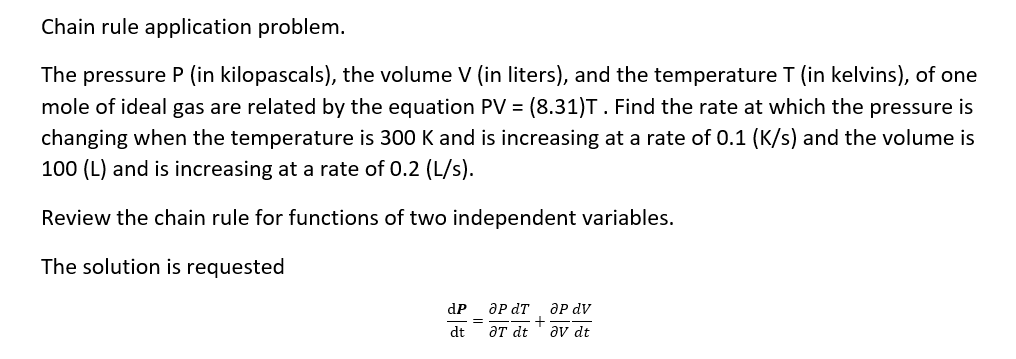
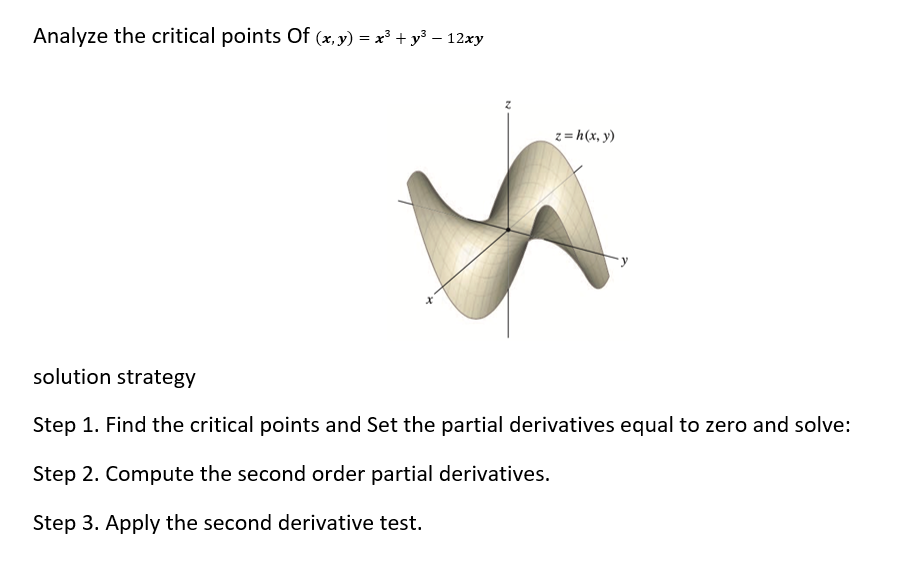
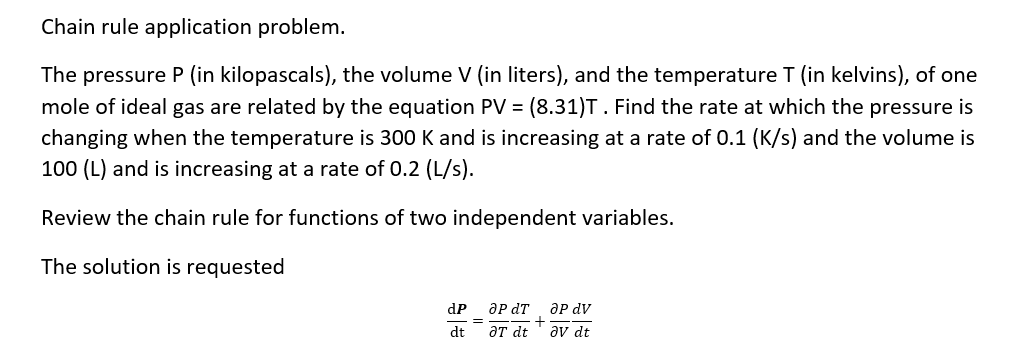
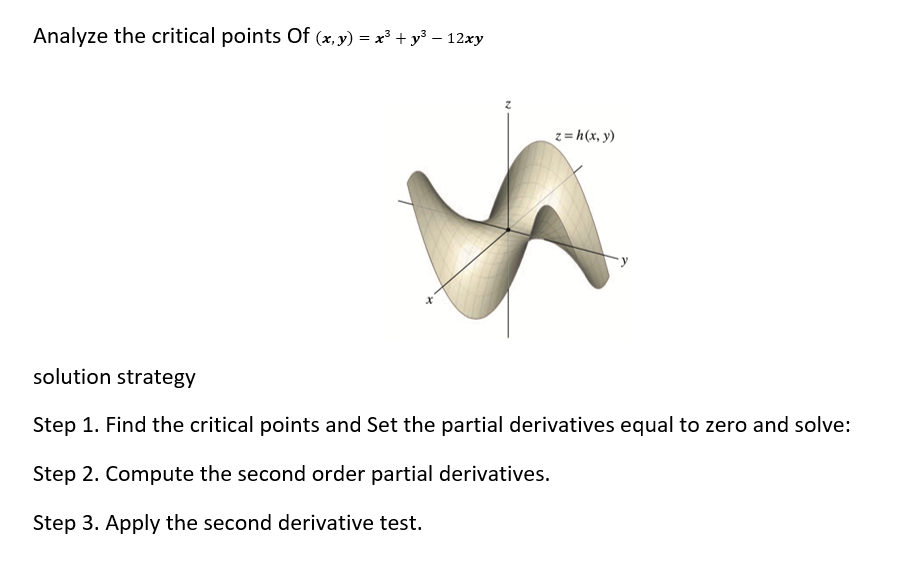
Chain rule application problem. The pressure P {in kilopascals), the volume V (in liters), and the temperature T (in kelvins), of one mole of ideal gas are related by the equation PV = (3.31)T. Find the rate at which the pressure is changing when the temperature is 300 K and is increasing at a rate of 0.1 \"([5] and the volume is 100 (L) and is increasing at a rate of 0.2 {Ms}. Review the chain rule for functions of two independent variables. The solution is requested dP_anT+anV dt _6Tdt aura: Analyze the critical points Of (any) = x3 + 3:3 12x3! solution strategy Step 1. Find the critical points and Set the partial derivatives equal to zero and solve: Step 2. Compute the second order partial derivatives. Step 3. Apply the second derivative test
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


