Question: Challenge Problem!!! Two 20.0g ice cubes at 17.0C are placed into 275g of water at 25.0C. Assuming no energy is transferred to or from the
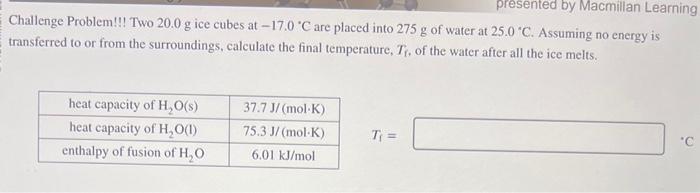
Challenge Problem!!! Two 20.0g ice cubes at 17.0C are placed into 275g of water at 25.0C. Assuming no energy is transferred to or from the surroundings, calculate the final temperature, Tf, of the water after all the ice melts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


