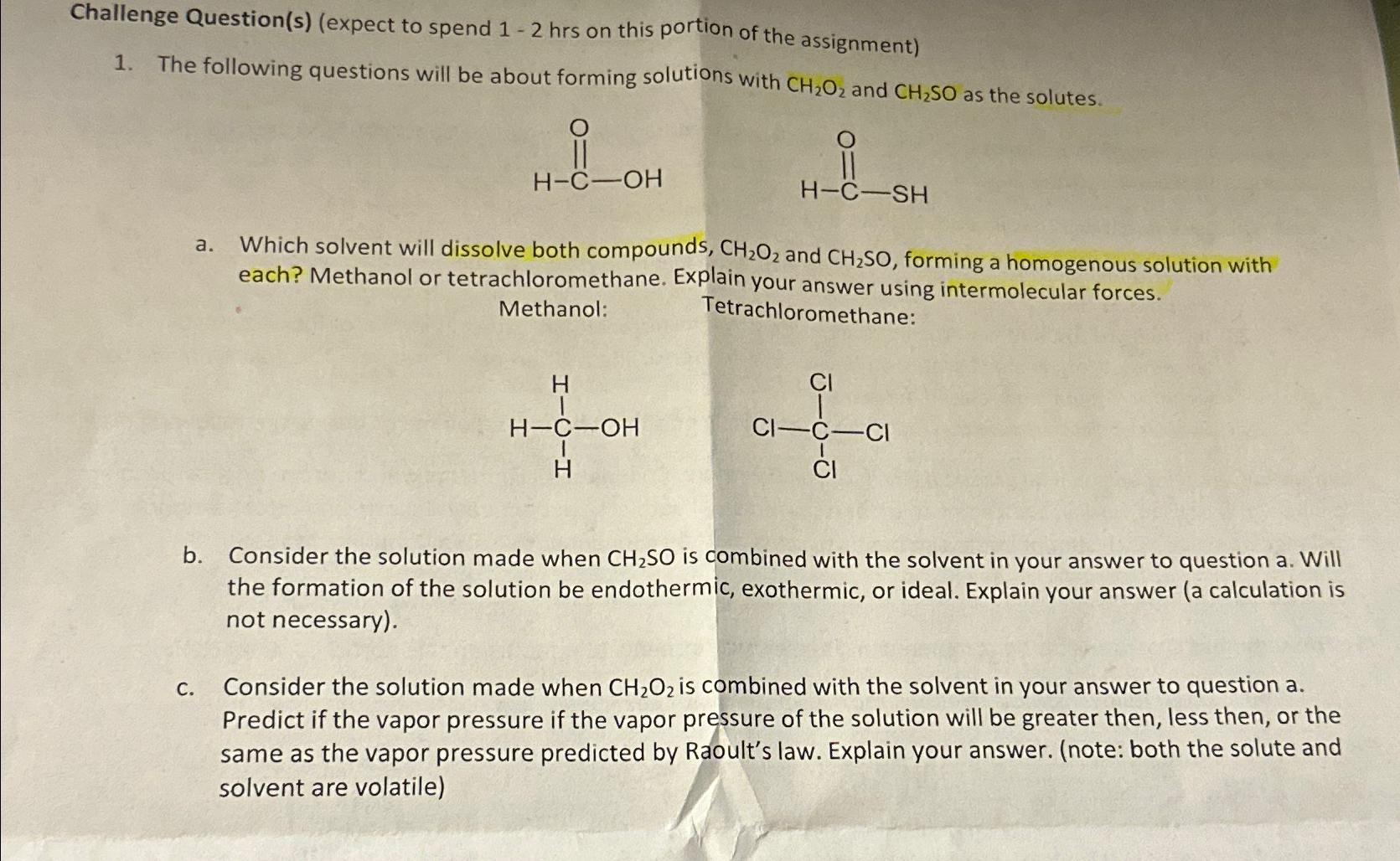
Question: Challenge Question ( s ) ( expect to spend 1 - 2 hrs on this portion of the assignment ) The following questions will be
Step by Step Solution
There are 3 Steps involved in it
Solution to Challenge Question Given Solutes CHO Formic acid CHSO Thioformic acid Solvents Methanol Tetrachloromethane CCl Part a Which solvent will d... View full answer

Get step-by-step solutions from verified subject matter experts


