Question: Challenges - Complete all challenges presented (A through E) - A. DRP for COVID-19 A domestic pharmaceutical firm (in your chosen country) has developed a

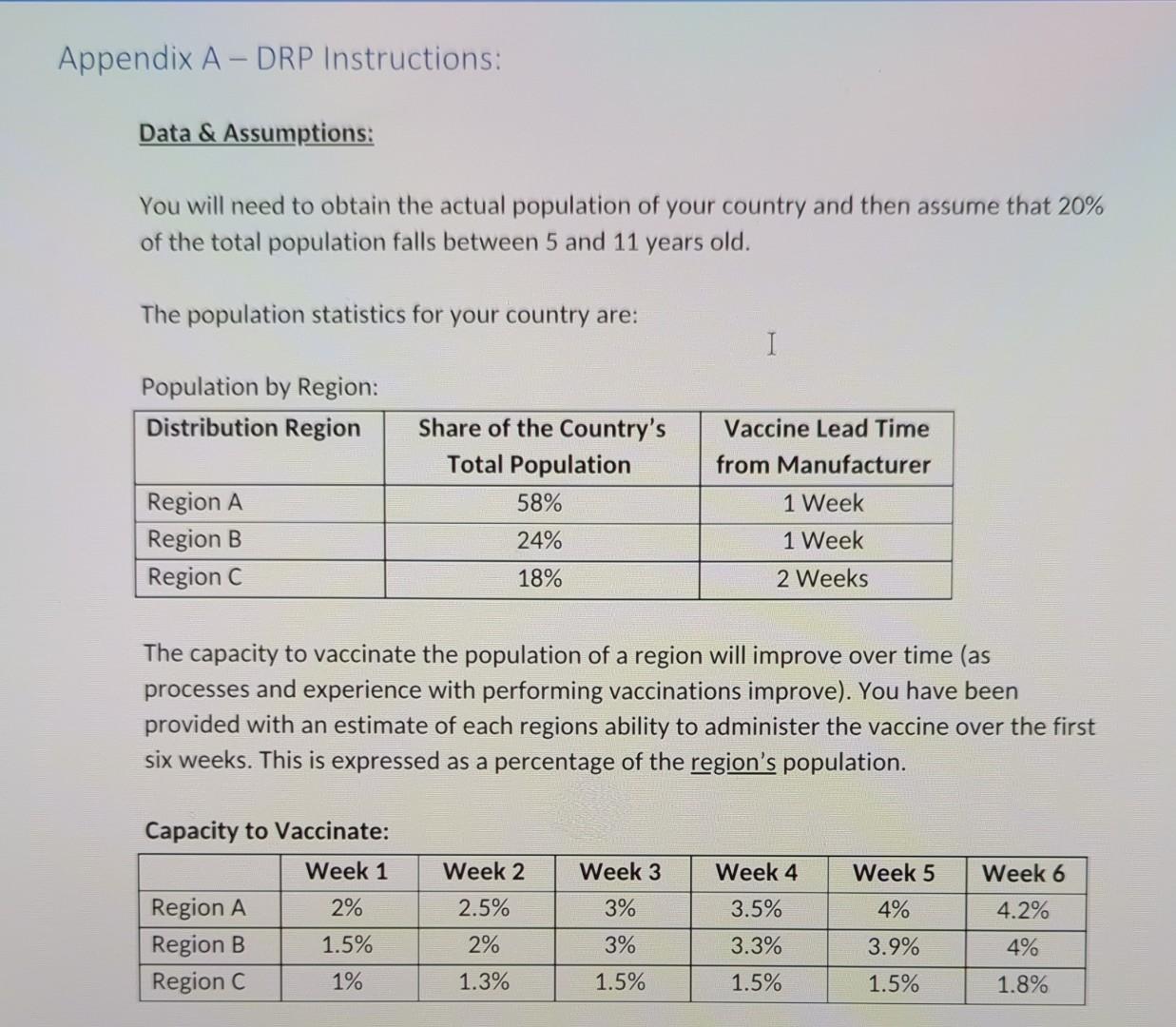
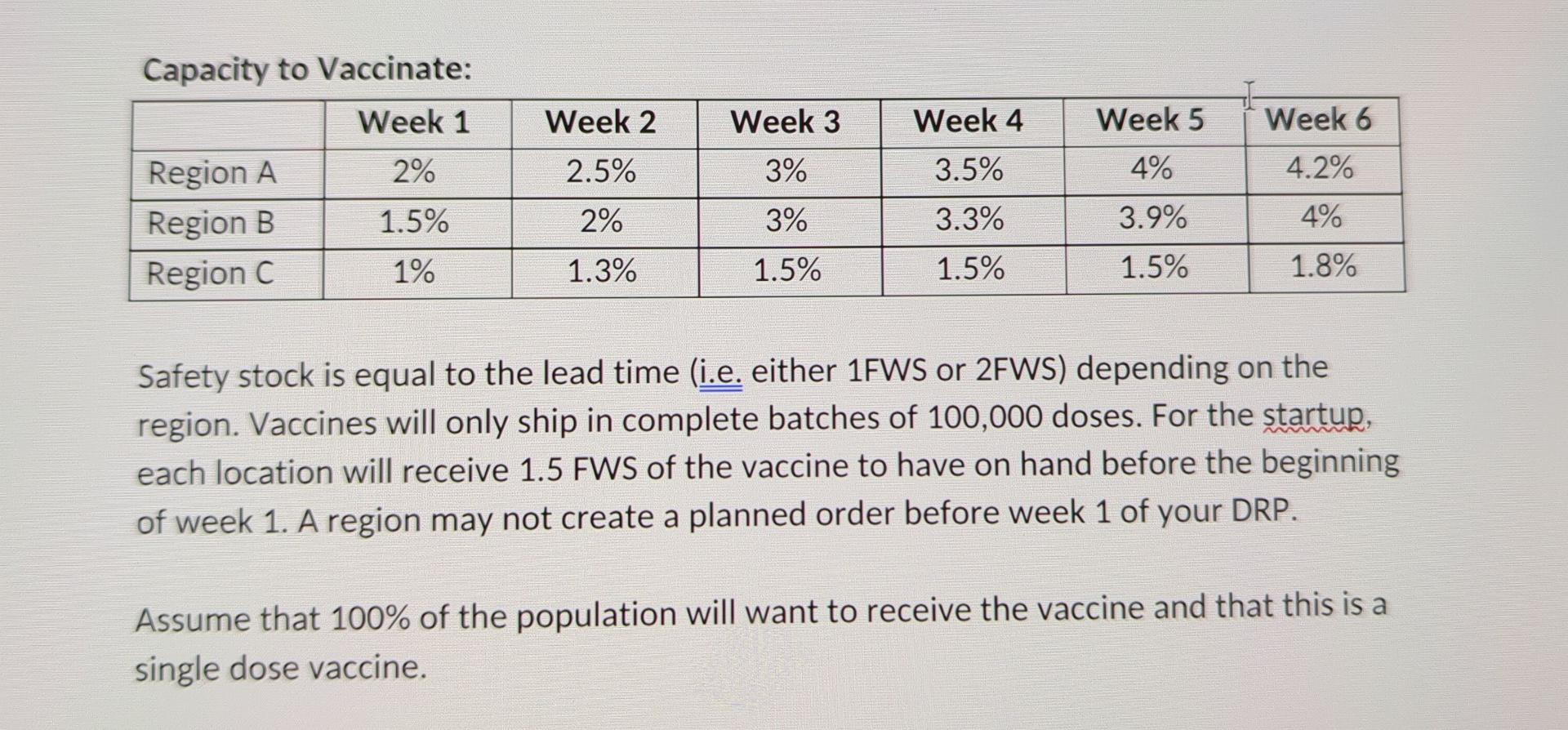




Challenges - Complete all challenges presented (A through E) - A. DRP for COVID-19 A domestic pharmaceutical firm (in your chosen country) has developed a COVID-19 Vaccine for children to be administered in your chosen country. You have been hired by the Federal Government of your chosen country work on the distribution of the vaccine to the population. To avoid regions within the country from hoarding the available vaccine supply, you have been tasked to develop a DRP that will provide factual information to aid in the distribution of the vaccine. The country has been divided into three geographic regions according to the statistics below. Your challenge is to complete a DRP plan for the first four week of the vaccinations that can be used by manufacturing to plan the volume of manufacturing needed. You will need to consider the capacity to vaccinate within each region as the independent demand. For this exercise, you simply need to consolidate the weekly demand that the manufacturer will need to produce i.e. planned production is equal to gross requirements for the final table in your DRP). See Appnedix A of this document for the necessary data & assumptions to complete your DRP. Appendix A - DRP Instructions: Data & Assumptions: You will need to obtain the actual population of your country and then assume that 20% of the total population falls between 5 and 11 years old. The population statistics for your country are: I Population by Region: Distribution Region Share of the Country's Total Population 58% Vaccine Lead Time from Manufacturer 1 Week Region A Region B Region C 24% 1 Week 18% 2 Weeks The capacity to vaccinate the population of a region will improve over time (as processes and experience with performing vaccinations improve). You have been provided with an estimate of each regions ability to administer the vaccine over the first six weeks. This is expressed as a percentage of the region's population. Capacity to Vaccinate: Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 2.5% 3% 4% 4.2% Region A Region B Region C 2% 1.5% 1% 3.5% 3.3% 2% 3% 3.9% 4% 1.3% 1.5% 1.5% 1.5% 1.8% Capacity to Vaccinate: Week 1 Week 2 Week 3 Week 4 Week 5 I Week 6 2% 2.5% 3% 3.5% 4% 4.2% Region A Region B Region C 1.5% 2% 3% 3.3% 3.9% 4% 1% 1.3% 1.5% 1.5% 1.5% 1.8% Safety stock is equal to the lead time (i.e. either 1FWS or 2FWS) depending on the region. Vaccines will only ship in complete batches of 100,000 doses. For the startup, each location will receive 1.5 FWS of the vaccine to have on hand before the beginning of week 1. A region may not create a planned order before week 1 of your DRP. Assume that 100% of the population will want to receive the vaccine and that this is a single dose vaccine. B. MPS for COVID-19 Vaccine You have been asked to also produce a MPS for the vaccine production for the first three weeks of the vaccination effort. The manufacturer would like to keep 1 FWS as the safety stock level and you are to assume that each dose will be made available for distribution (ATP is equal to PAB). The manufacturer has one primary constraint - doses must be manufactured in batches of 200,000 at a time. The manufacturer will operate seven days a week and feels confident that they will be able to produce the demand requirements each week. Note: For this challenge you will likely complete the MPS using a spreadsheet application. You are required to correctly paste your completed tables into this space and if required, change the page formatting (landscape vs portrait). Your tables should be legible, clean, and professionally organized and presented. Tables that are not properly formatted and presented will have mark reductions applied. C. Threats and Opportunities with DRP and MPS You have been invited to a strategy meeting prior to the roll-out of the vaccine where the Director of the project the project would like each team to comment on any issues or opportunities that exist in their areas. As a member of the supply chain team, discuss any treats or opportunities that exist based on your preparation of the DRP and MPS using your specialized knowledge of supply chain practices. D. Ensuring the Inventory is Accurate The Director of the program is concerned about the potential for inventory inaccuracies to cause potential public outrage and backlash against the program. Specifically, the Director is concerned with the each three of the region's ability to maintain accurate inventory records and is asking you to develop recommendations for each region on how to best manage the accuracy of their inventory. E. Actual Manufacturing In preparation of your DRP and MPS you have learned that the actual amount that can be produced by the design capacity of the production system is actually 600,000 at a time. Using our course concepts, describe why there the manufacturer would not produce to this volume and if you would anticipate fluctuations in the actual production amount. Support your opinions. Challenges - Complete all challenges presented (A through E) - A. DRP for COVID-19 A domestic pharmaceutical firm (in your chosen country) has developed a COVID-19 Vaccine for children to be administered in your chosen country. You have been hired by the Federal Government of your chosen country work on the distribution of the vaccine to the population. To avoid regions within the country from hoarding the available vaccine supply, you have been tasked to develop a DRP that will provide factual information to aid in the distribution of the vaccine. The country has been divided into three geographic regions according to the statistics below. Your challenge is to complete a DRP plan for the first four week of the vaccinations that can be used by manufacturing to plan the volume of manufacturing needed. You will need to consider the capacity to vaccinate within each region as the independent demand. For this exercise, you simply need to consolidate the weekly demand that the manufacturer will need to produce i.e. planned production is equal to gross requirements for the final table in your DRP). See Appnedix A of this document for the necessary data & assumptions to complete your DRP. Appendix A - DRP Instructions: Data & Assumptions: You will need to obtain the actual population of your country and then assume that 20% of the total population falls between 5 and 11 years old. The population statistics for your country are: I Population by Region: Distribution Region Share of the Country's Total Population 58% Vaccine Lead Time from Manufacturer 1 Week Region A Region B Region C 24% 1 Week 18% 2 Weeks The capacity to vaccinate the population of a region will improve over time (as processes and experience with performing vaccinations improve). You have been provided with an estimate of each regions ability to administer the vaccine over the first six weeks. This is expressed as a percentage of the region's population. Capacity to Vaccinate: Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 2.5% 3% 4% 4.2% Region A Region B Region C 2% 1.5% 1% 3.5% 3.3% 2% 3% 3.9% 4% 1.3% 1.5% 1.5% 1.5% 1.8% Capacity to Vaccinate: Week 1 Week 2 Week 3 Week 4 Week 5 I Week 6 2% 2.5% 3% 3.5% 4% 4.2% Region A Region B Region C 1.5% 2% 3% 3.3% 3.9% 4% 1% 1.3% 1.5% 1.5% 1.5% 1.8% Safety stock is equal to the lead time (i.e. either 1FWS or 2FWS) depending on the region. Vaccines will only ship in complete batches of 100,000 doses. For the startup, each location will receive 1.5 FWS of the vaccine to have on hand before the beginning of week 1. A region may not create a planned order before week 1 of your DRP. Assume that 100% of the population will want to receive the vaccine and that this is a single dose vaccine. B. MPS for COVID-19 Vaccine You have been asked to also produce a MPS for the vaccine production for the first three weeks of the vaccination effort. The manufacturer would like to keep 1 FWS as the safety stock level and you are to assume that each dose will be made available for distribution (ATP is equal to PAB). The manufacturer has one primary constraint - doses must be manufactured in batches of 200,000 at a time. The manufacturer will operate seven days a week and feels confident that they will be able to produce the demand requirements each week. Note: For this challenge you will likely complete the MPS using a spreadsheet application. You are required to correctly paste your completed tables into this space and if required, change the page formatting (landscape vs portrait). Your tables should be legible, clean, and professionally organized and presented. Tables that are not properly formatted and presented will have mark reductions applied. C. Threats and Opportunities with DRP and MPS You have been invited to a strategy meeting prior to the roll-out of the vaccine where the Director of the project the project would like each team to comment on any issues or opportunities that exist in their areas. As a member of the supply chain team, discuss any treats or opportunities that exist based on your preparation of the DRP and MPS using your specialized knowledge of supply chain practices. D. Ensuring the Inventory is Accurate The Director of the program is concerned about the potential for inventory inaccuracies to cause potential public outrage and backlash against the program. Specifically, the Director is concerned with the each three of the region's ability to maintain accurate inventory records and is asking you to develop recommendations for each region on how to best manage the accuracy of their inventory. E. Actual Manufacturing In preparation of your DRP and MPS you have learned that the actual amount that can be produced by the design capacity of the production system is actually 600,000 at a time. Using our course concepts, describe why there the manufacturer would not produce to this volume and if you would anticipate fluctuations in the actual production amount. Support your opinions
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


