Question: 6) Ethyl ether is made by dehydration of ethyl alcohol in the presence of sulfuric acid at 140C: 2 C2H5OH > C2H5OC2H5 + H20
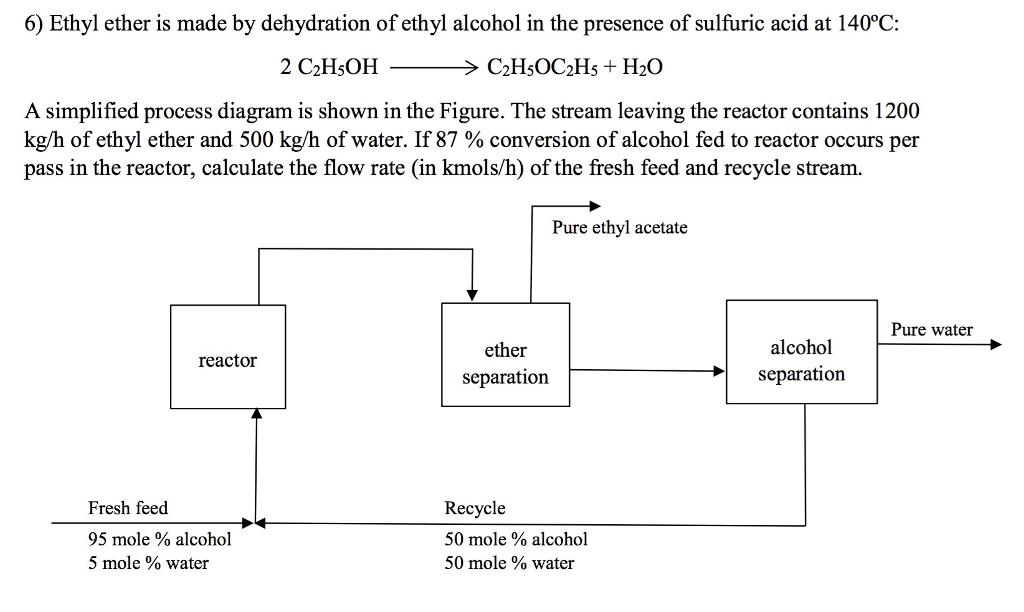
6) Ethyl ether is made by dehydration of ethyl alcohol in the presence of sulfuric acid at 140C: 2 C2H5OH > C2H5OC2H5 + H20 A simplified process diagram is shown in the Figure. The stream leaving the reactor contains 1200 kg/h of ethyl ether and 500 kg/h of water. If 87 % conversion of alcohol fed to reactor occurs per pass in the reactor, calculate the flow rate (in kmols/h) of the fresh feed and recycle stream. Pure ethyl acetate Pure water ether alcohol reactor separation separation Fresh feed Recycle 95 mole % alcohol 50 mole % alcohol 5 mole % water 50 mole % water
Step by Step Solution
3.39 Rating (152 Votes )
There are 3 Steps involved in it
V Water is formed as a product from the reaction By the ... View full answer

Get step-by-step solutions from verified subject matter experts


