Question: Chapter 15 Algorithmic Question 16 Part A The decomposition of dinitrogen pentoxide is described by the following chemical equation 2N2O5(g)4NO2(g)+O2(g) If the rate of appearance
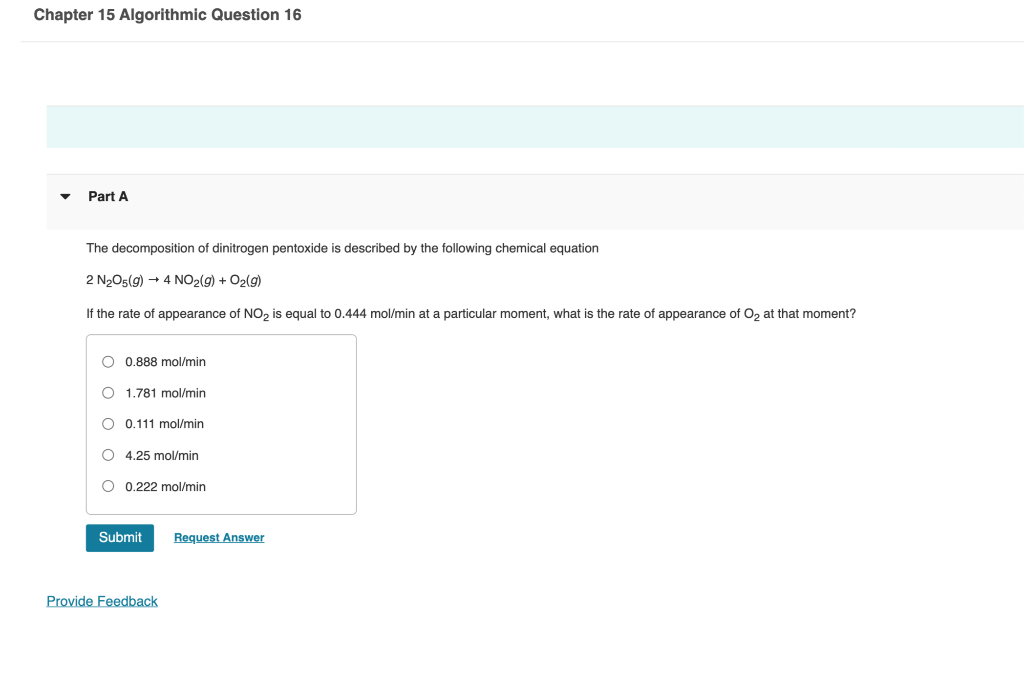
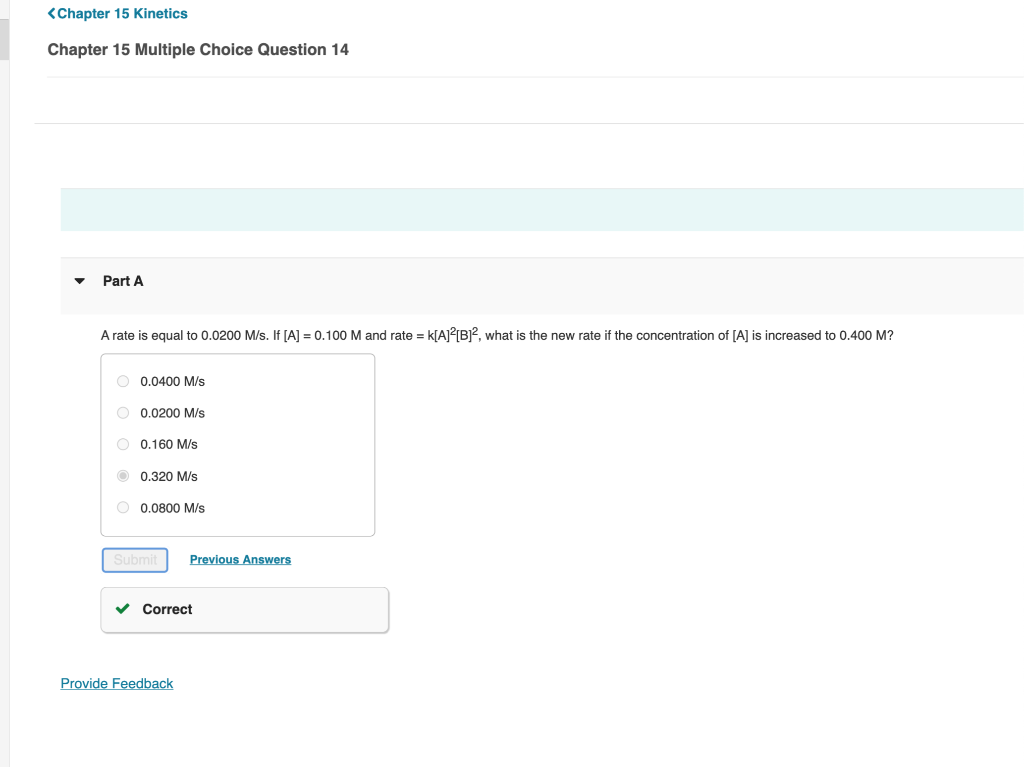
Chapter 15 Algorithmic Question 16 Part A The decomposition of dinitrogen pentoxide is described by the following chemical equation 2N2O5(g)4NO2(g)+O2(g) If the rate of appearance of NO2 is equal to 0.444mol/min at a particular moment, what is the rate of appearance of O2 at that moment? 0.888mol/min 1.781mol/min 0.111mol/min 4.25mol/min 0.222mol/min A rate is equal to 0.0200M/s. If [A]=0.100M and rate =k[A]2[B]2, what is the new rate if the concentration of [A] is increased to 0.400M ? 0.0400M/s0.0200M/s0.160M/s0.320m/s0.0800M/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


