Question: chapter 9, question 9. please answer completely! 9. (-/10 Points CHANGPCHEM1 9.P.011. DETAILS MY NOTES ASK YOUR TEACHER PRACTICE ANOTHER An electrochemical cell consists of
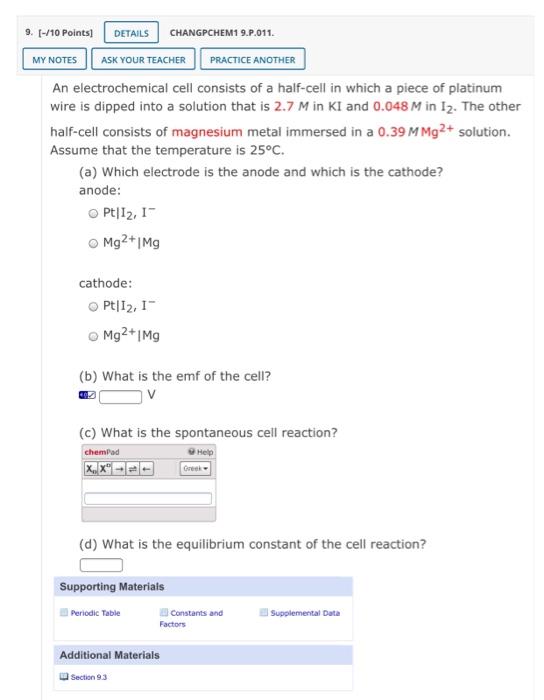
9. (-/10 Points CHANGPCHEM1 9.P.011. DETAILS MY NOTES ASK YOUR TEACHER PRACTICE ANOTHER An electrochemical cell consists of a half-cell in which a piece of platinum wire is dipped into a solution that is 2.7 M in KI and 0.048 M in 12. The other half-cell consists of magnesium metal immersed in a 0.39 M Mg2+ solution. Assume that the temperature is 25C. (a) Which electrode is the anode and which is the cathode? anode: Pt|12, 1- Mg2+Mg cathode: Pt|12,1" Mg2+IME (b) What is the emf of the cell? (c) What is the spontaneous cell reaction? chemad Help (d) What is the equilibrium constant of the cell reaction? Supporting Materials Periodic Table Constants and Factors Supplemental Data Additional Materials Section 93 9. (-/10 Points CHANGPCHEM1 9.P.011. DETAILS MY NOTES ASK YOUR TEACHER PRACTICE ANOTHER An electrochemical cell consists of a half-cell in which a piece of platinum wire is dipped into a solution that is 2.7 M in KI and 0.048 M in 12. The other half-cell consists of magnesium metal immersed in a 0.39 M Mg2+ solution. Assume that the temperature is 25C. (a) Which electrode is the anode and which is the cathode? anode: Pt|12, 1- Mg2+Mg cathode: Pt|12,1" Mg2+IME (b) What is the emf of the cell? (c) What is the spontaneous cell reaction? chemad Help (d) What is the equilibrium constant of the cell reaction? Supporting Materials Periodic Table Constants and Factors Supplemental Data Additional Materials Section 93
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


