Question: Check my work 11 Enter your answer in the provided boxes. Nail polish remover containing acetone was spilled in a room 7.63 m *5.68 m




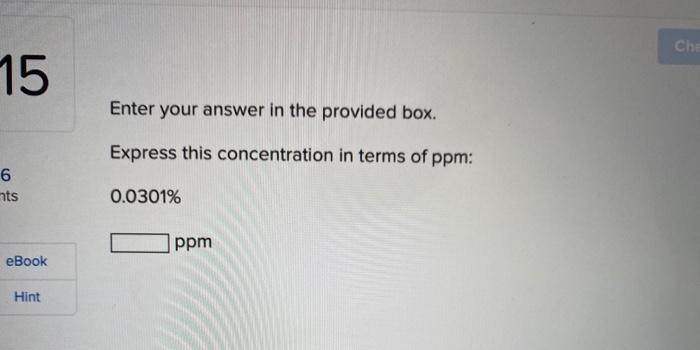
Check my work 11 Enter your answer in the provided boxes. Nail polish remover containing acetone was spilled in a room 7.63 m *5.68 m *5.16 m. S Measurements indicated that 9,750 mg of acetone evaporated. Calculate the acetone concentration in micrograms per cubic meter. Book Hint x 10 Hg m (Enter your answer in scientific notation.) Check my wo 2 Click in the answer box to activate the palette. Provide the correct name for the following molecular compound: CO2 ook Check my 13 Enter your answer in the provided box. How many carbon atoms does the hydrocarbon ethane contain? s Number of carbons = eBook Hint Checkm 14 Enter your answer in the provided box. 6 nts To burn 1 molecule of C5H12 to form CO2 and H2O (complete combustion), how many molecules of O2 are required? eBook molecules Hint Che 15 Enter your answer in the provided box. Express this concentration in terms of ppm: 6 nts 0.0301% ppm eBook Hint
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


