Question: Chem 1 4 0 5 : Electronic configuration Bohr's planetary model of the atom is drawn below to show 6 energy levels of the electron.
Chem : Electronic configuration
Bohr's planetary model of the atom is drawn below to show energy levels of the electron.
Draw the following transitions on the model:
Which transition corresponds with
a The least amount of energy absorbed
b The largest amount of energy emitted?
c What energy level is the ground state of this electron lowest energy level
Draw the shape of following orbitals:
a one s orbital shape
b three porbitals shapes
c five dorbitals shapes
Draw Lewis dot structures of the following elements
Al
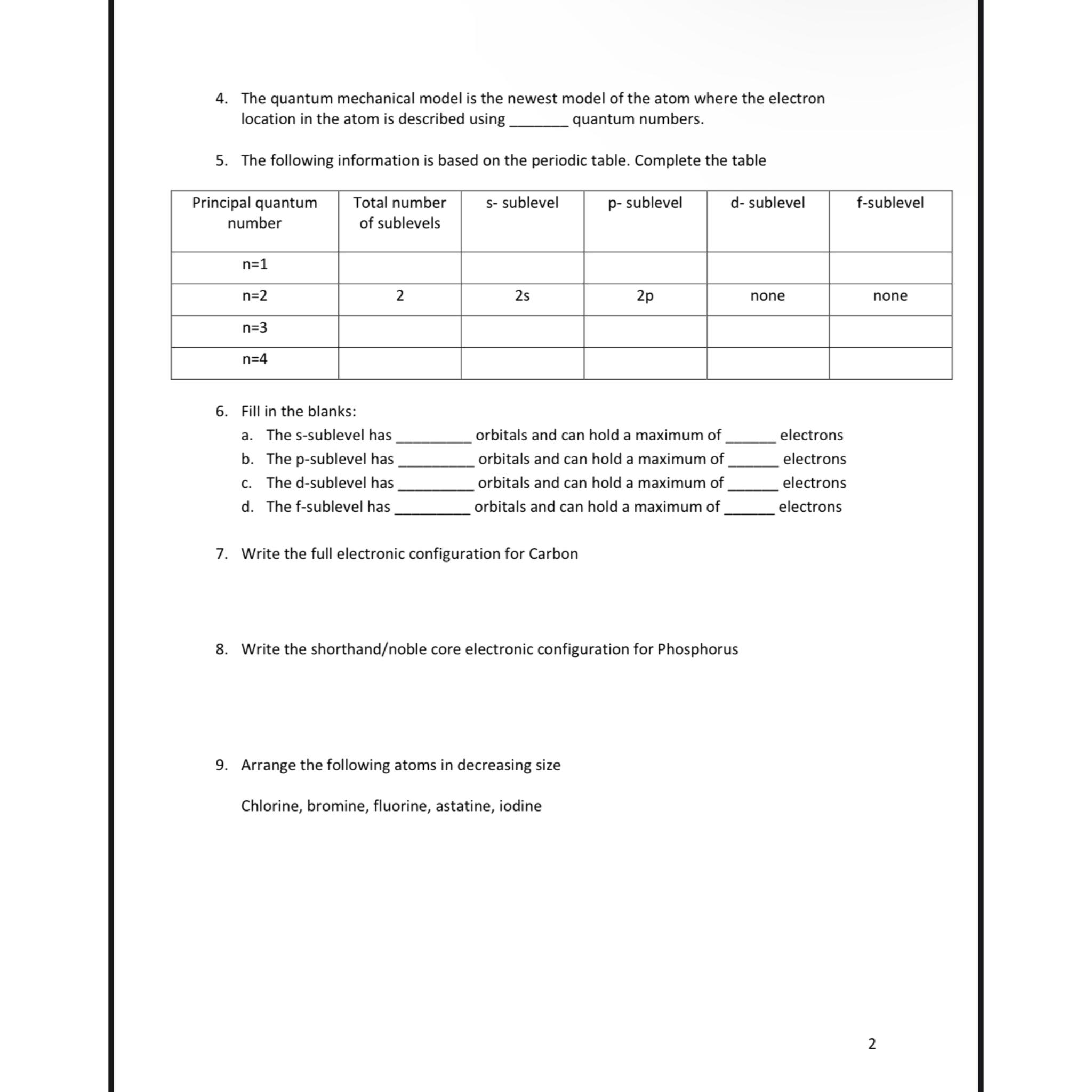
The quantum mechanical model is the newest model of the atom where the electron location in the atom is described using quantum numbers.
The following information is based on the periodic table. Complete the table
tabletablePrincipal quantumnumbertableTotal numberof sublevelss sublevel,psublevel,d sublevel,fsublevelnone,none
Fill in the blanks:
a The ssublevel has
orbitals and can hold a maximum of
b The psublevel has
c The dsublevel has
d The fsublevel has orbitals and can hold a maximum of rrbitals and can hold a maximum of orbitals and can hold a maximum of
electrons electrons electrons electrons
Write the full electronic configuration for Carbon
Write the shorthandnoble core electronic configuration for Phosphorus
Arrange the following atoms in decreasing size
Chlorine, bromine, fluorine, astatine, iodine
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


