Question: 2 1. (1) Calculate AS for the reactions given below, using the data given in the table. Say why AS is not a valid
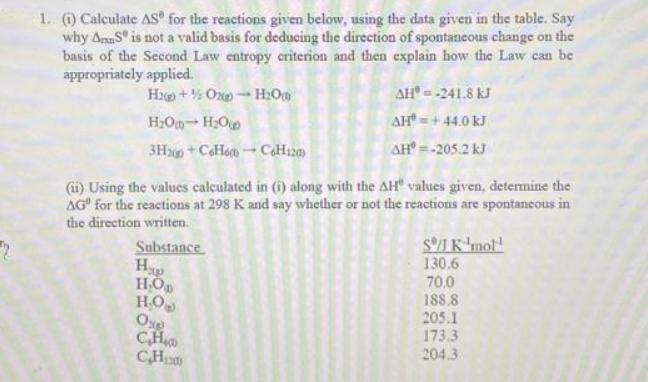


2 1. (1) Calculate AS for the reactions given below, using the data given in the table. Say why AS is not a valid basis for deducing the direction of spontaneous change on the basis of the Second Law entropy criterion and then explain how the Law can be appropriately applied. H2+%Ox-H00 HO-HO 3H2+CH6-CH120) (ii) Using the values calculated in (i) along with the AH" values given, determine the AG" for the reactions at 298 K and say whether or not the reactions are spontaneous in the direction written. Substance H HO H.O AH* = -241.8 kJ AH = +44.0 kJ AH* = -205.2 kJ Oxg C.H. CH S/J K mol 130.6 70,0 188.8 205.1 173.3 204.3 2. By considering the sign and magnitude of the enthalpic and entropic components of the Gibbs energy in the reactions given below, determine the range of temperatures over which the reactions will be spontaneous. (1) NH4Cl() NH3(g) + HCl(g) (ii) 302(g) 203(g) (iii) 2HS(g) + SO2(g) 3S()+ 2H2Om) (iv) H2(g) + Brz 2HBr(s) 1 AH = 176 kJ mol, AS = 285 J K-mol- AH-285 kJ mol, AS = -137 J K-mol- AH = -233 kJ mol-, AS = -424 J K mol- AH-72.8 kJ mol, AS - 114 J K-mol- 3. (1) Write an equation to represent the vaporization of water. (ii) Considering that S for HOg) is 70.0 J K whereas that for HO S is 188.8 J K. Calculate the free energy change for this process at 25 C if AH for this process is 44.0 kJ. (iii) By considering the relation relationship between AG and equilibrium constant, K, calculate the partial pressure of water vapour at 25 C and also 50 C 4. Given that Ag+(aq) +e Ag(s) Zn+(aq) +2e=Zn(s) (i)Write the overall cell reaction. (ii) Calculate the standard cell potential and free energy change at 298 K. (F 96500 C/mol) C E/V +0.80 E%/ V=-0.76 (iii) Sketch a fully labelled diagram of the electrochemical cell in which this reaction would occur spontaneously. (iv) Based on the diagram in (iii), explain the direction of electron flow and the importance of the slat bridge. (v) Using the fact that AG=-nFE and AG - -RTln K, derive a law which connects the cell potential to the activities of electrolyte species in solution.
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
Based on the photos provided it appears that you have a set of questions related to thermodynamics particularly the calculation of entropy changes S free energy changes G and understanding electrochem... View full answer

Get step-by-step solutions from verified subject matter experts


