Question: CHEM 251 Problem Set #1 Inorganic Chemstry Please help!!! Dinitrogen molecular orbital diagram. Draw lines to orbital #2 consistent with s-p mixing. Draw two combinations
CHEM 251 Problem Set #1 Inorganic Chemstry Please help!!!
Dinitrogen molecular orbital diagram. Draw lines to orbital #2 consistent with s-p mixing. Draw two combinations of atomic orbitals with appropriate phasing that would result in a non-bonding orbital. Identify the HOMO by number. 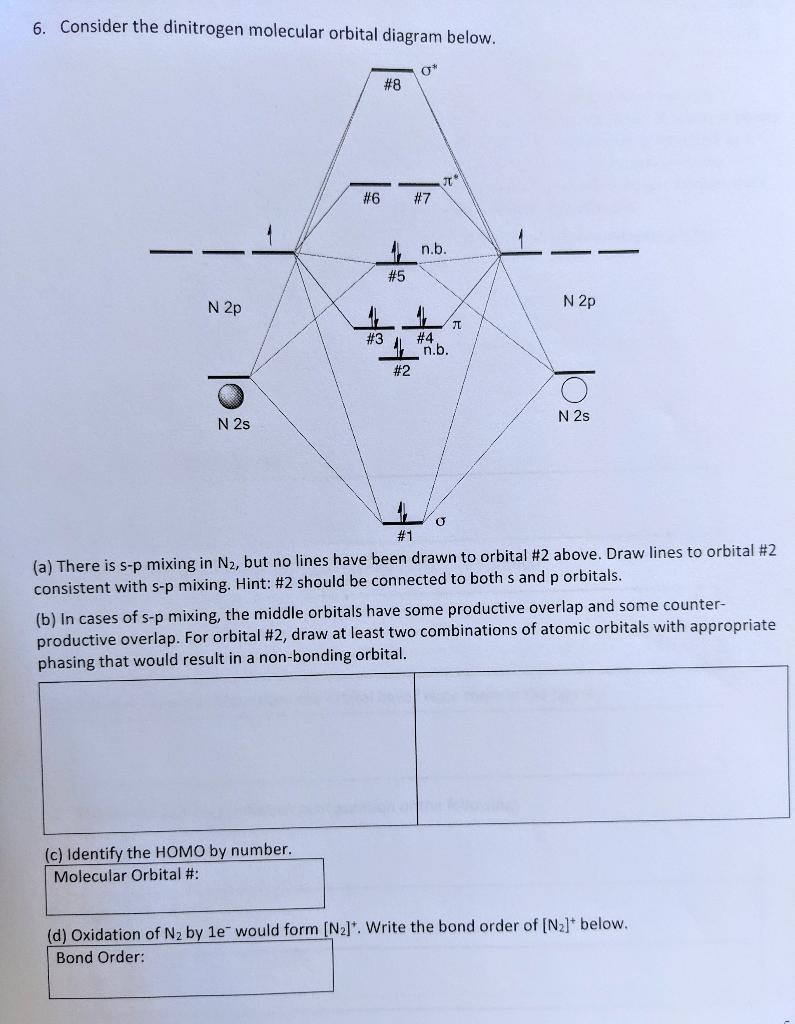
6. Consider the dinitrogen molecular orbital diagram below. O" #8 T #6 #7 n.b. #5 N 2p N 2p #3 11 #4. 1 n.b. #2 loi N 2s N 2s #1 (a) There is s-p mixing in N2, but no lines have been drawn to orbital #2 above. Draw lines to orbital #2 consistent with s-p mixing. Hint: #2 should be connected to both s and p orbitals. (b) In cases of s-p mixing, the middle orbitals have some productive overlap and some counter- productive overlap. For orbital #2, draw at least two combinations of atomic orbitals with appropriate phasing that would result in a non-bonding orbital. (c) Identify the HOMO by number. Molecular Orbital #: (d) Oxidation of Nz by le would form (N2]*. Write the bond order of [N2]* below. Bond Order
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


