Question: Chem - B 4 / May 2 0 1 8 0 4 - Chem - A 3 , Mass Transfer Operations December 2 0 1
ChemBMay
ChemA Mass Transfer Operations
December
PART A: ANSWER ONE OF QUESTIONS
Note: Four questions constitute a complete paper with one from Part one from Part B and two from Part C
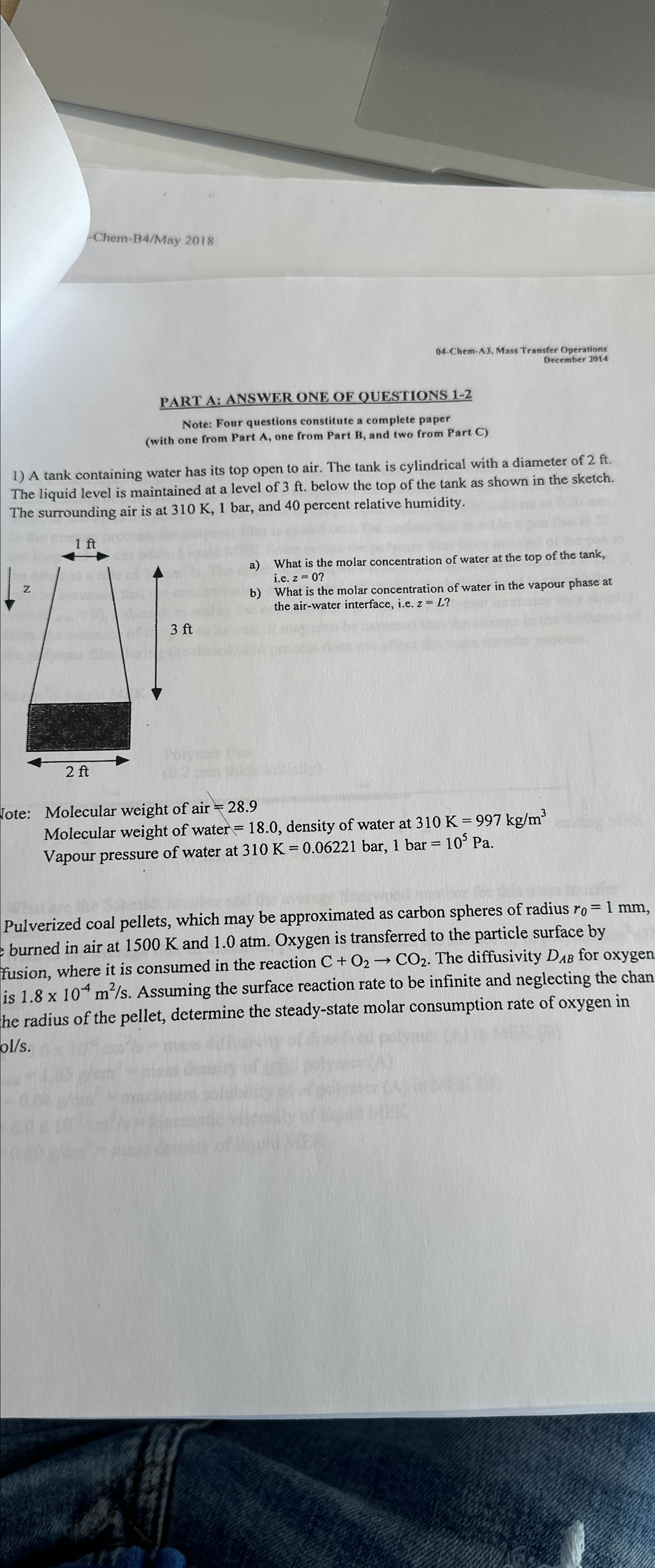
A tank containing water has its top open to air. The tank is cylindrical with a diameter of The liquid level is maintained at a level of below the top of the tank as shown in the sketch. The surrounding air is at and percent relative humidity.
a What is the molar concentration of water at the top of the tank, ie
b What is the molar concentration of water in the vapour phase at the airwater interface, ie
Jote: Molecular weight of air
Molecular weight of water density of water at
Vapour pressure of water at
Pulverized coal pellets, which may be approximated as carbon spheres of radius burned in air at and atm. Oxygen is transferred to the particle surface by fusion, where it is consumed in the reaction The diffusivity for oxygen is Assuming the surface reaction rate to be infinite and neglecting the chan he radius of the pellet, determine the steadystate molar consumption rate of oxygen in
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


