Question: Chemical Engineering Reaction (Problem Question) The relationship between the speed expression of any reaction studied and the transformation is shown below: FA0=a.X2+b.X+10rAAA Which reactor (PAR
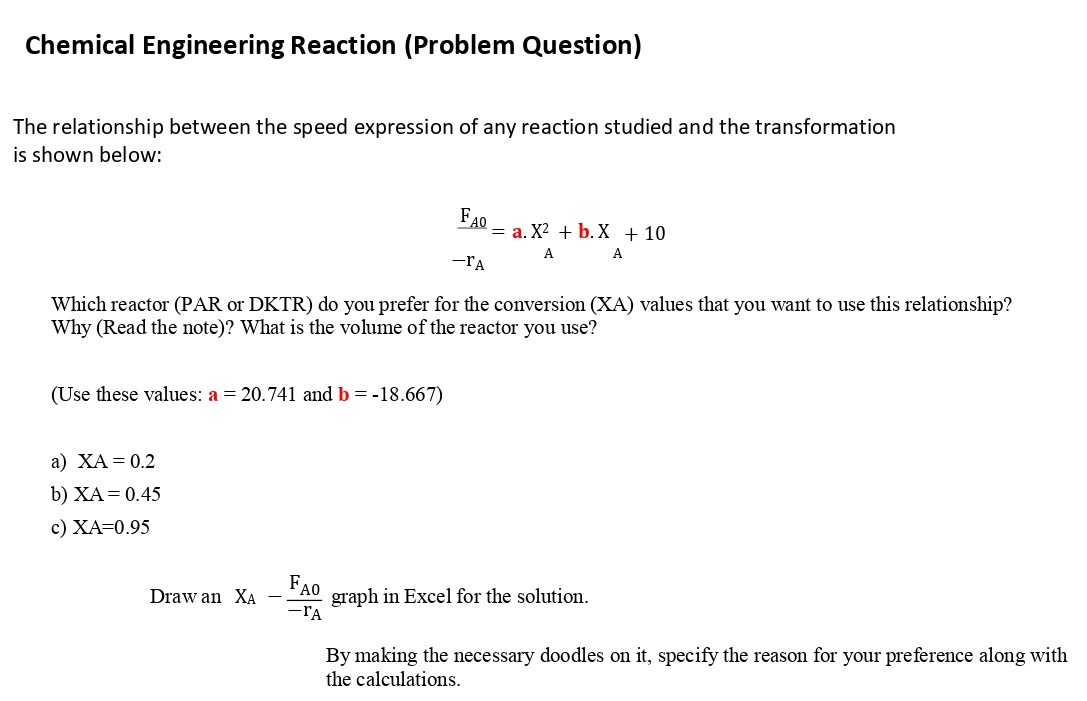
Chemical Engineering Reaction (Problem Question) The relationship between the speed expression of any reaction studied and the transformation is shown below: FA0=a.X2+b.X+10rAAA Which reactor (PAR or DKTR) do you prefer for the conversion (XA) values that you want to use this relationship? Why (Read the note)? What is the volume of the reactor you use? (Use these values: a=20.741 and b=18.667 ) a) XA=0.2 b) XA=0.45 c) XA=0.95 Draw an XArAFA0 graph in Excel for the solution. By making the necessary doodles on it, specify the reason for your preference along with the calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


