Question: CHEMICAL ENGINEERING SEPARATION PROCESS A continuous distillation column is designed to separate a liquid mixture with a boiling point containing 45 mol% methanol and 55mol%
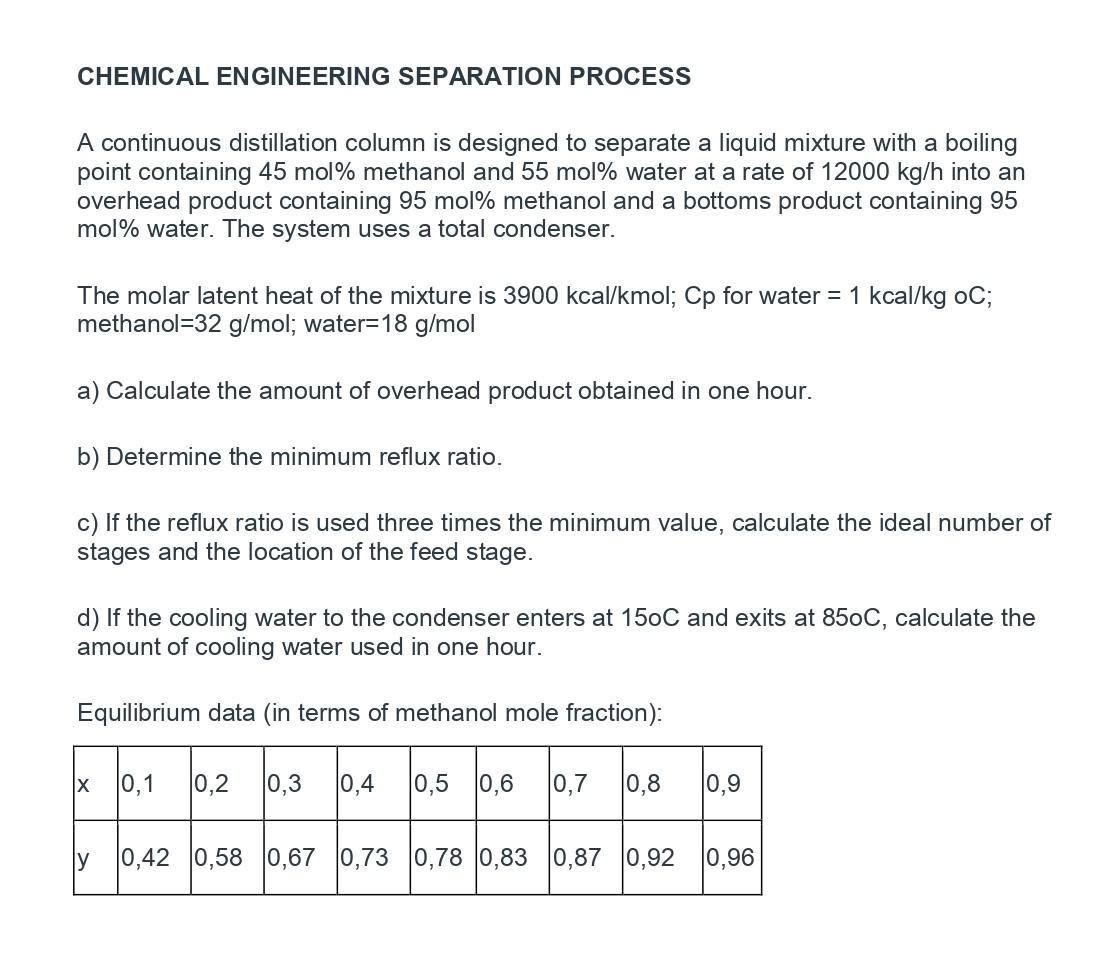
CHEMICAL ENGINEERING SEPARATION PROCESS A continuous distillation column is designed to separate a liquid mixture with a boiling point containing 45 mol\% methanol and 55mol% water at a rate of 12000kg/h into an overhead product containing 95 mol\% methanol and a bottoms product containing 95 mol\% water. The system uses a total condenser. The molar latent heat of the mixture is 3900kcal/kmol;Cp for water =1kcal/kgoC; methanol =32g/mol; water =18g/mol a) Calculate the amount of overhead product obtained in one hour. b) Determine the minimum reflux ratio. c) If the reflux ratio is used three times the minimum value, calculate the ideal number of stages and the location of the feed stage. d) If the cooling water to the condenser enters at 150C and exits at 850C, calculate the amount of cooling water used in one hour. Equilibrium data (in terms of methanol mole fraction)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


