Question: Chemical Process Principle Help with this question please. I need the solution ASAP. Thnkyou so much Question 4 (20 Marks) (a) A stream of ethane
Chemical Process Principle
Help with this question please. I need the solution ASAP. Thnkyou so much
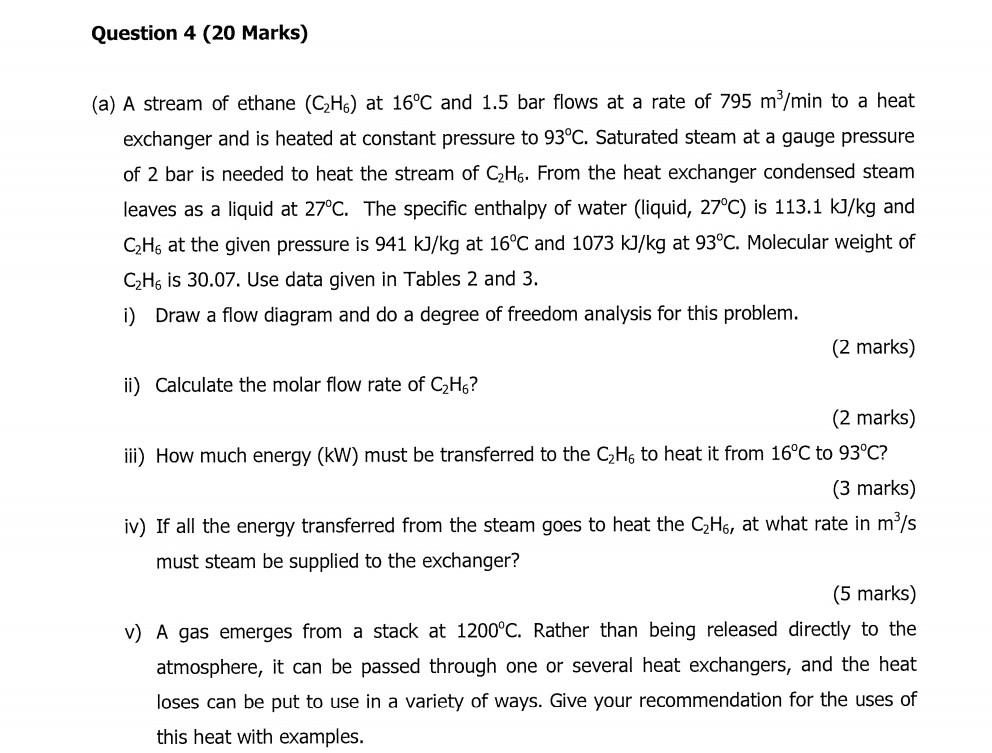
Question 4 (20 Marks) (a) A stream of ethane (C2H6) at 16C and 1.5 bar flows at a rate of 795 m/min to a heat exchanger and is heated at constant pressure to 93C. Saturated steam at a gauge pressure of 2 bar is needed to heat the stream of C2H6. From the heat exchanger condensed steam leaves as a liquid at 27C. The specific enthalpy of water (liquid, 27C) is 113.1 kJ/kg and CzHg at the given pressure is 941 kJ/kg at 16C and 1073 kJ/kg at 93C. Molecular weight of CH. is 30.07. Use data given in Tables 2 and 3. i) Draw a flow diagram and do a degree of freedom analysis for this problem. (2 marks) ii) Calculate the molar flow rate of CzH6? (2 marks) iii) How much energy (kW) must be transferred to the C2H6 to heat it from 16C to 93C? (3 marks) iv) If all the energy transferred from the steam goes to heat the CzHg, at what rate in m/s must steam be supplied to the exchanger? (5 marks) V) A gas emerges from a stack at 1200C. Rather than being released directly to the atmosphere, it can be passed through one or several heat exchangers, and the heat loses can be put to use in a variety of ways. Give your recommendation for the uses of this heat with examples
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


