Question: chemistry I've calculated the expected mass over and over and Idk what im doing wrong. Reevaluate your calculations. Did you report your data to the
chemistry
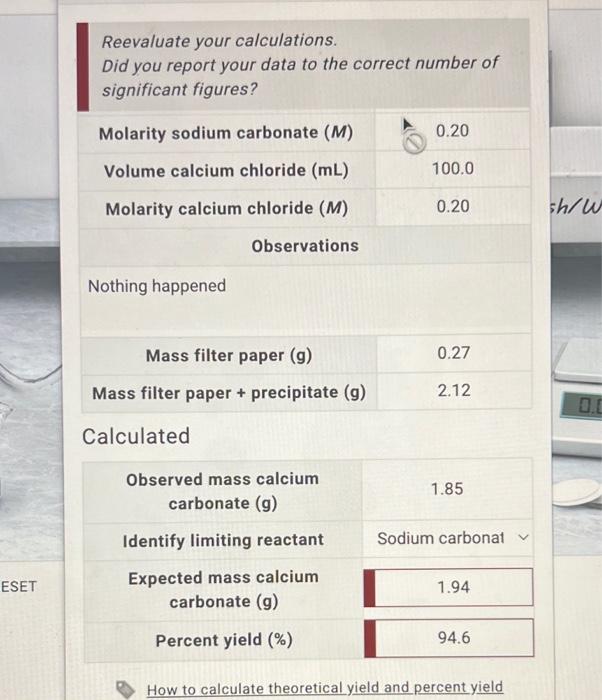
I've calculated the expected mass over and over and Idk what im doing wrong.
Reevaluate your calculations. Did you report your data to the correct number of significant figures? Molaritysodiumcarbonate(M)Volumecalciumchloride(mL)Molaritycalciumchloride(M)0.20100.00.20 Observations Nothing happened \begin{tabular}{c|c} Mass filter paper (g) & 0.27 \\ \hline Mass filter paper + precipitate (g) & 2.12 \\ \hline \end{tabular} Calculated Observed mass calcium carbonate (g) 1.85 Identify limiting reactant Sodium carbonat Expected mass calcium carbonate (g) 1.94 Percent yield (%) 94.6 How to calculate theoretical yield and percent yield
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


