Question: Write the full (all electrons) electron configuration for an atom of gallium (Ga). Then state the number of valance electrons the atom contains, and
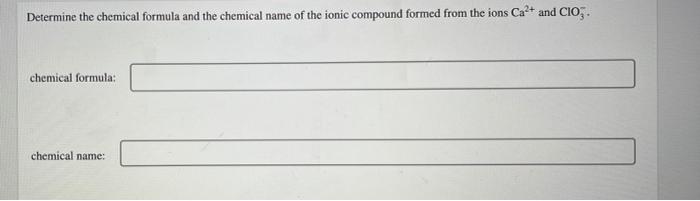
Write the full (all electrons) electron configuration for an atom of gallium (Ga). Then state the number of valance electrons the atom contains, and in which energy level (n value) the valence electrons reside. Determine the chemical formula and the chemical name of the ionic compound formed from the ions Cat and CIO. chemical formula: chemical name:
Step by Step Solution
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Part1 Gallium electronic configuration Writing the electron configuration code for gallium we get 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 1 In numerical order the electronic configuration for gallium i... View full answer

Get step-by-step solutions from verified subject matter experts



