Question: Chlorocyclopentadiene does not ionize easily in solution. It will ionize only when treated with a very strong Lewis acid such as antimony pentafluoride (SbF5). The
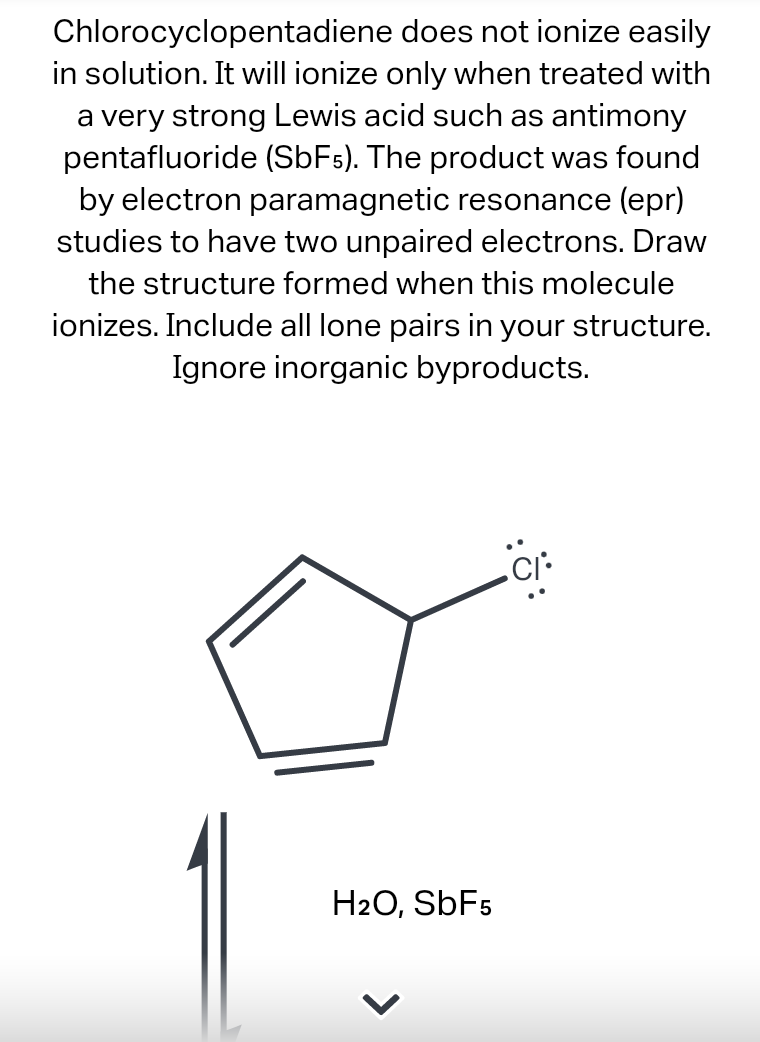
Chlorocyclopentadiene does not ionize easily in solution. It will ionize only when treated with a very strong Lewis acid such as antimony pentafluoride (SbF5). The product was found by electron paramagnetic resonance (epr) studies to have two unpaired electrons. Draw the structure formed when this molecule ionizes. Include all lone pairs in your structure. Ignore inorganic byproducts. CI: H2O, SbF5
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


