Question: Choose the correct statement; Select one I is a good nucleophile in ligand substitution reactions and a good leaving group H2O is a good nuoleophile


3, then the oxidation state of](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6d1d880464_60866f6d1d805616.jpg)




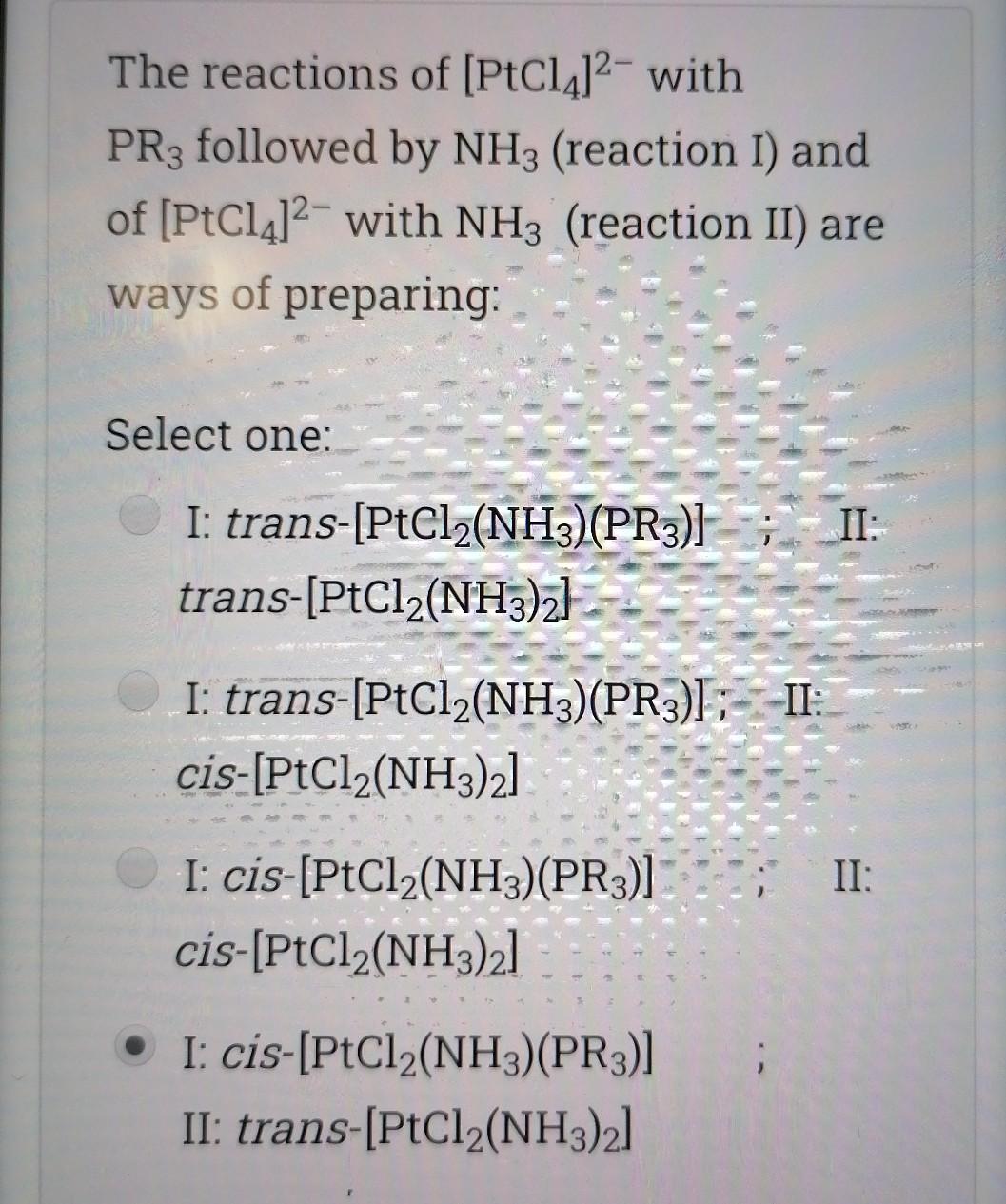
![Clear my choice The reactions of (PtCl4]2- with PR3 followed by NH3](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6d1de9908b_61466f6d1de18b75.jpg)
![(reaction I) and of [PtCl4]2- with NH3 (reaction II) are ways of](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6d1df74503_61466f6d1dee3f72.jpg)
![preparing: Select one: II: I: trans-[PtCl2(NH3)(PR3)}; trans-[PtCl (NH3)2] II: I: trans-[PtCl2(NH3)(PR3)];--II: cis-[PtCl2(NH3)2]](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6d1e05cad6_61566f6d1dfb5789.jpg)
![I: cis-[PtCl2(NH3)(PR3)] cis-[PtCl2(NH3)2] I: cis-[PtCl2(NH3)(PR3)] II: trans-[PtCl2(NH3)2] Which of the following statements](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6d1e1491c5_61666f6d1e0b0912.jpg)
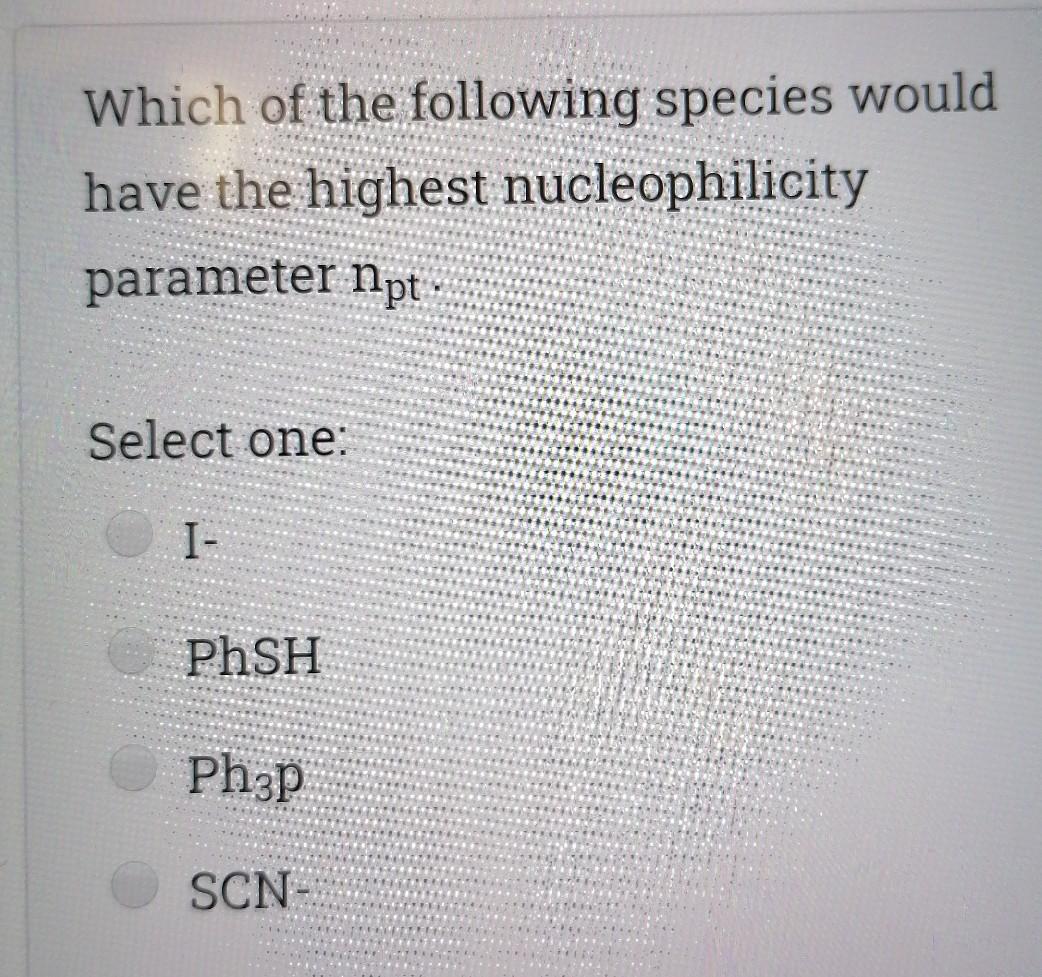
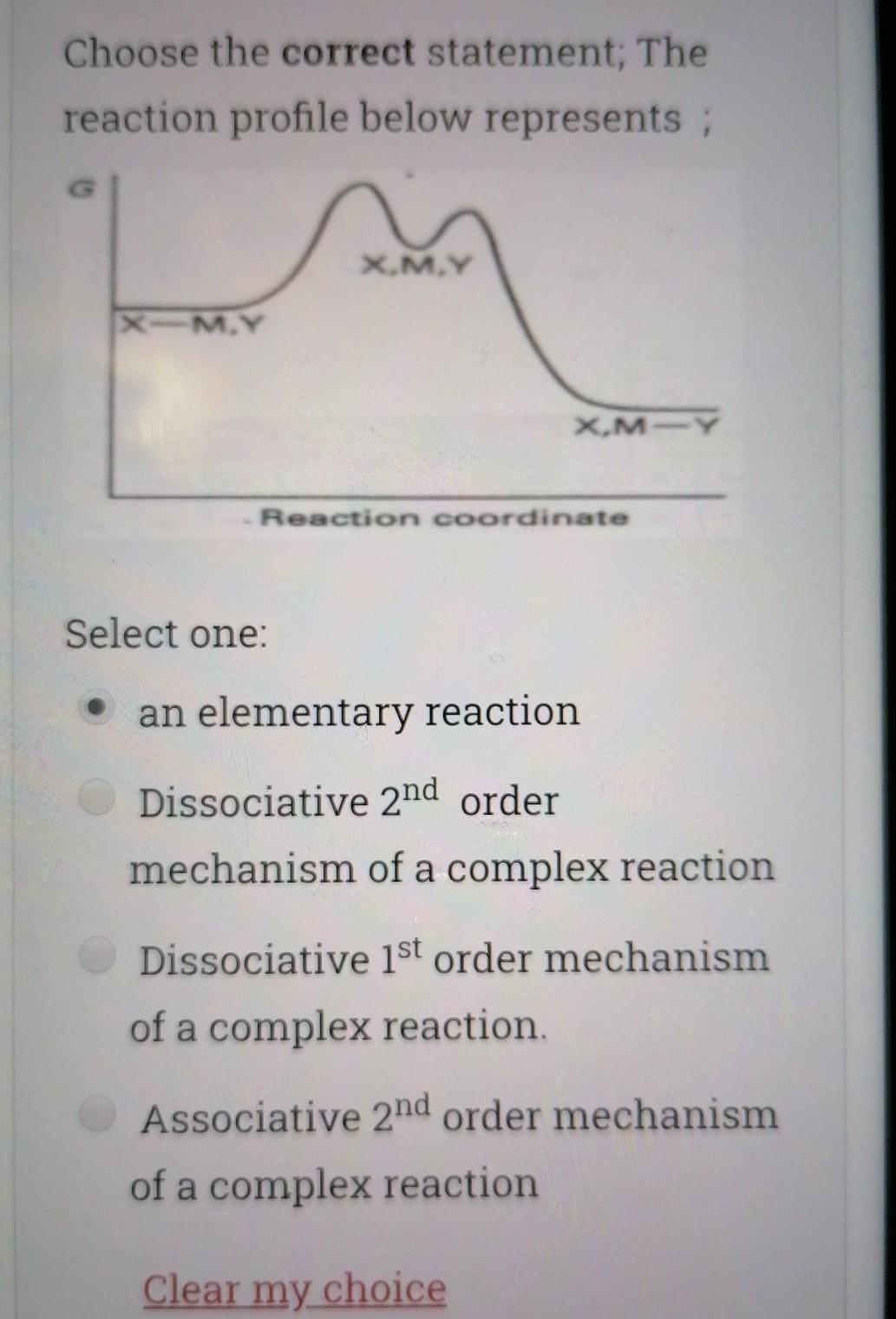
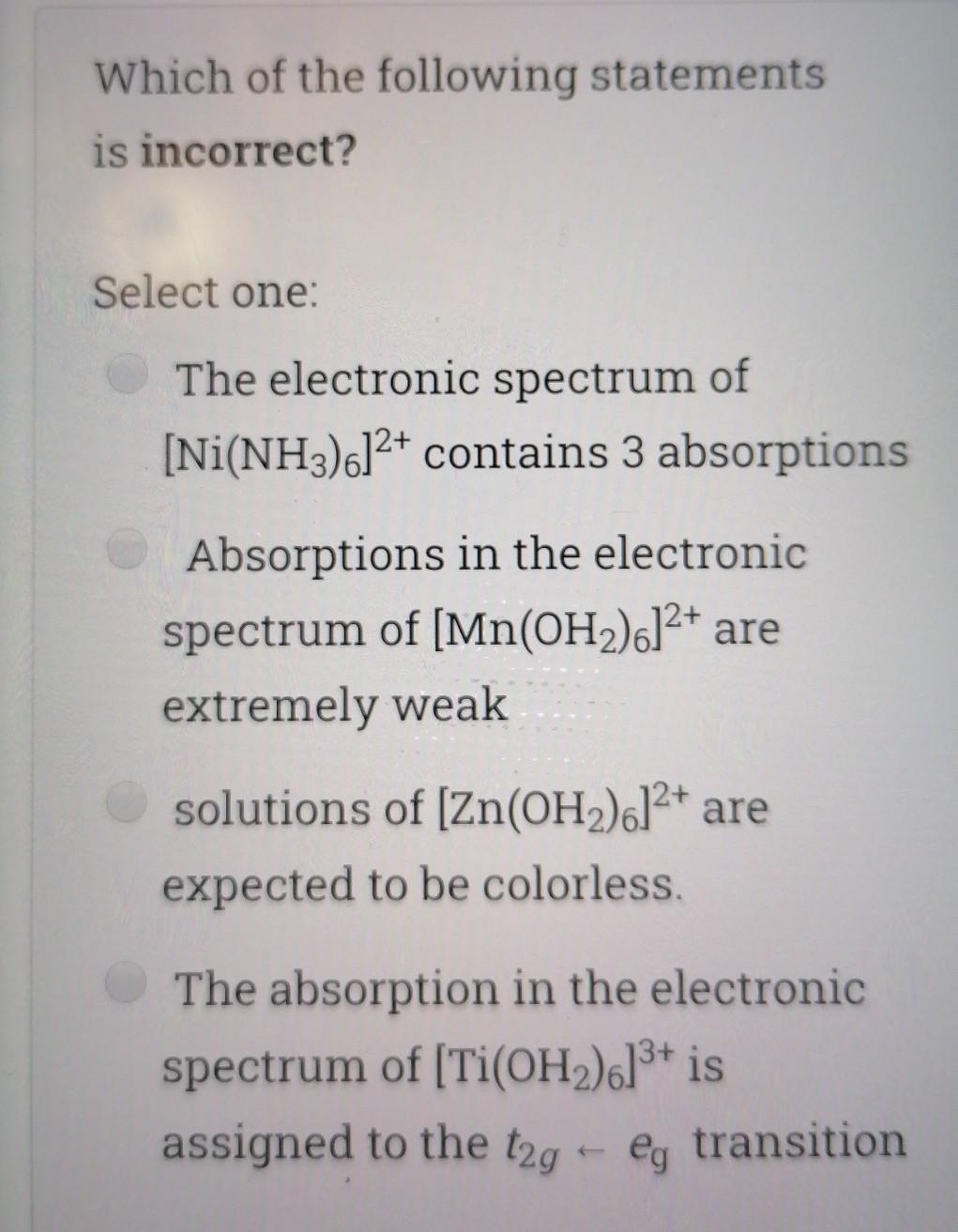
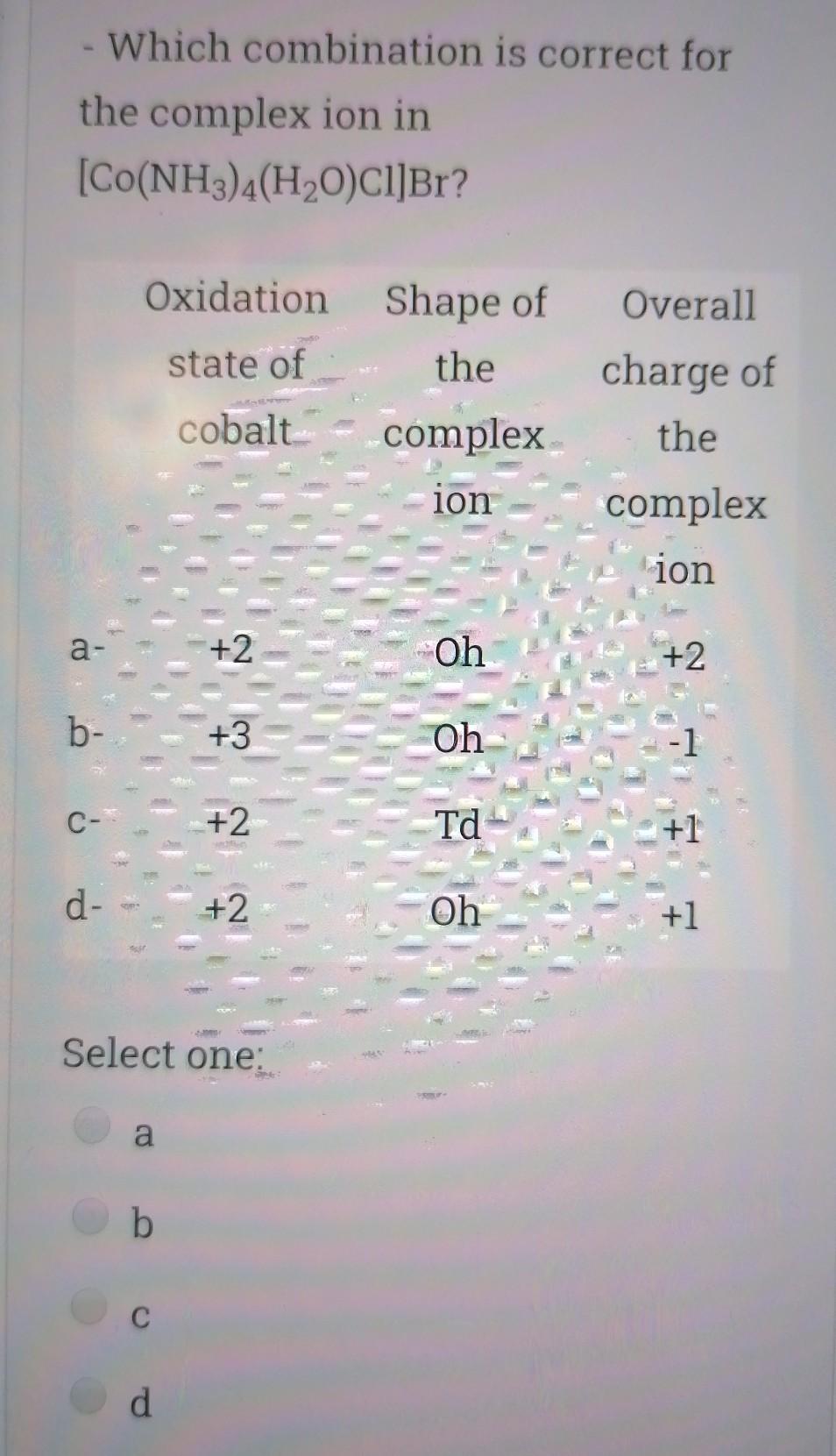
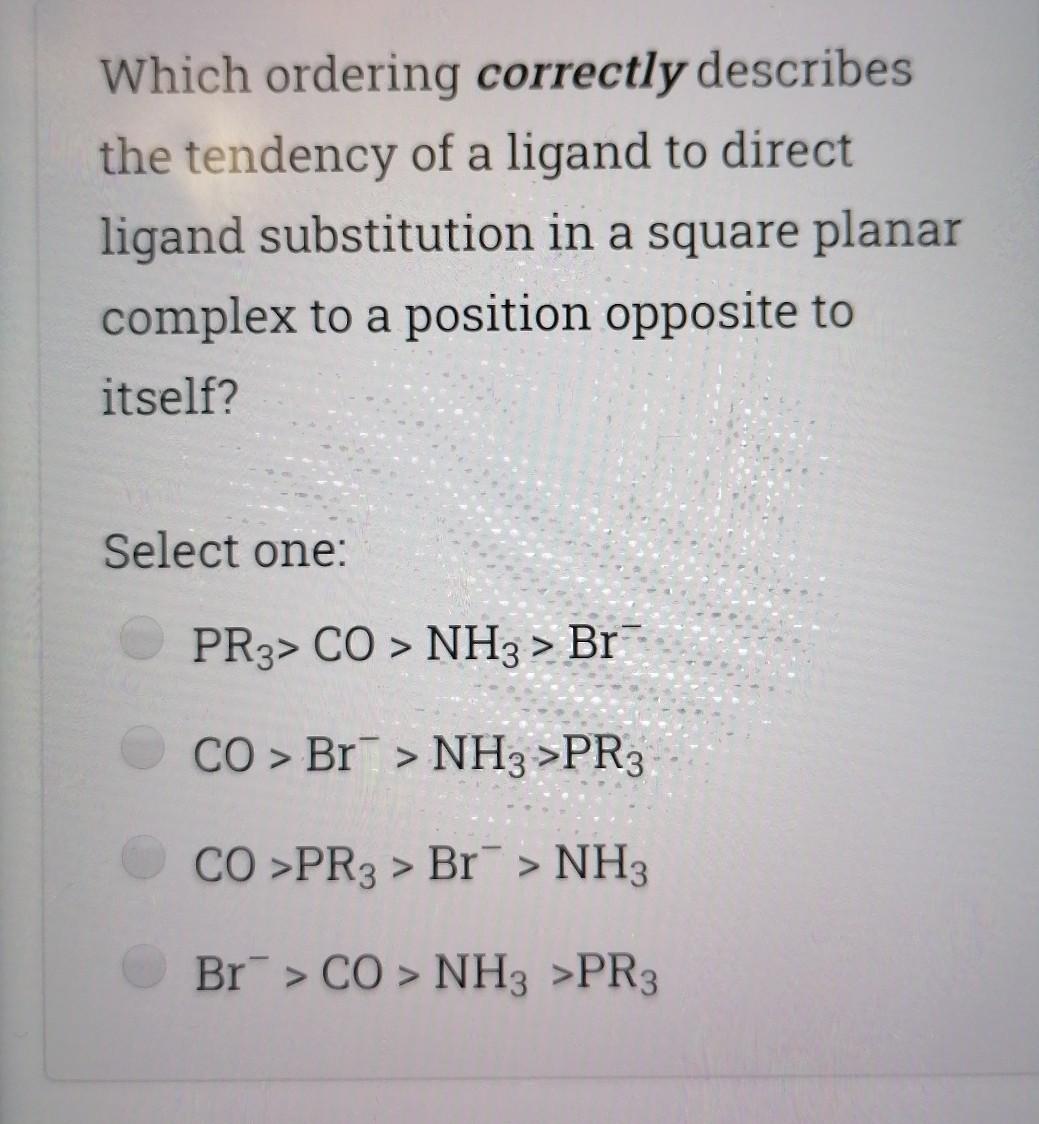
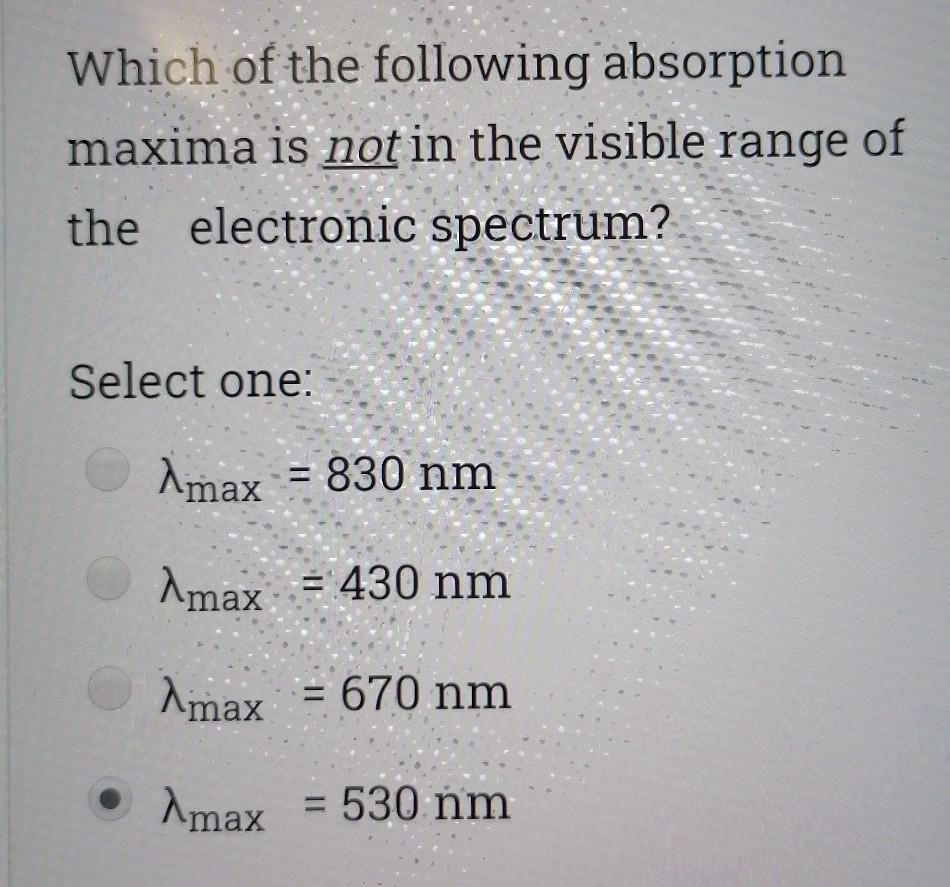
Choose the correct statement; Select one I is a good nucleophile in ligand substitution reactions and a good leaving group H2O is a good nuoleophile in ligand substitution reactions and a good leaving group CO is a good nucleophile in ligand substitution reactions and a good leaving group Interchange mechanism is a 2nd order substitution reaction which takes place in sq.planar complexes 1. Which of the following species would have the highest nucleophilicity parameter npt Select one: I- PhSH Ph3P SCN- Suppose you have the coordination compound : [Rh(en)3 ](ClO4)3, then the oxidation state of Rh, the d-electron count, and the magnetic properties for this complex would be : Select one: + 6d3 paramagnetic +3 db diamagnetic 0, d7 paramagnetic +3, do paramagnetic Choose the correct statement, The reaction profile below represents ; X.MY X-M.Y X.M-Y Reaction coordinate Select one: an elementary reaction Dissociative 2nd order mechanism of a complex reaction Dissociative 1st order mechanism of a complex reaction. Associative 2nd order mechanism of a complex reaction Clear my choice The reactions of (PtCl4]2- with PR3 followed by NH3 (reaction I) and of [PtCl4]2- with NH3 (reaction II) are ways of preparing: Select one: II: I: trans-[PtCl2(NH3)(PR3)}; trans-[PtCl (NH3)2] II: I: trans-[PtCl2(NH3)(PR3)];--II: cis-[PtCl2(NH3)2] I: cis-[PtCl2(NH3)(PR3)] cis-[PtCl2(NH3)2] I: cis-[PtCl2(NH3)(PR3)] II: trans-[PtCl2(NH3)2] Which of the following statements is incorrect? Select one: The electronic spectrum of [Ni(NH3).]2+ contains 3 absorptions Absorptions in the electronic spectrum of [Mn(OH2)6]2+ are extremely weak solutions of (Zn(OH2)6]2+ are expected to be colorless. The absorption in the electronic spectrum of (TI(OH2)6]3+ is assigned to the tag - eg transition - Which combination is correct for the complex ion in [Co(NH3)4(H20)Cl]Br? Oxidation Overall Shape of the state of charge of the cobalt complex ion complex ion a- +2 Oh +2 b- +3 Oh C- +2 Td d +2 Oh +1 Select one: a b d Choose the correct statement Select one: An elementary step has 0 intermediates & O transition states An elementary step has 0 intermediates & 1 transition state a complex reaction involves 1 intermediate & 1 transition state. An elementary step has 1 intermediate & 1 transition state Which ordering correctly describes the tendency of a ligand to direct ligand substitution in a square planar complex to a position opposite to itself? Select one: PR3> CO > NH3 > Br CO > Br > NH3 >PR3 CO >PR3 > Br"> NH3 Br > CO > NH3 >PR3 Choose the correct statement; Metal - PR3 p- bonding takes place between; Select one: a filled metal t2g d-orbital & an empty d- orbital of P atom a filled metal tag d-orbital & an empty p-orbital of P atom a filled metal eg d-orbital & an empty p-orbital of P atom a filled metal eg d-orbital & an empty d- orbital of P atom Which of the following absorption maxima is not in the visible range of the electronic spectrum? Select one: Imax = 830 nm max Imax = 430 nm Imax = 670 nm Imax = 530 nm Which statement is incorrect about [TI(OH2)613+? Select one: It is octahedral Solutions of the ion are coloured It is paramagnetic Considering only the d-orbitals, crystal field theory is consistent with a ground state electronic configuration of egt2g. Which of the following complexes would you expect to be colorless? i. [Y(H20)6]3+ ii: [Cr(en)313+ iii.(Cd(NH3)4]2+ iv. [CO(NH3)612+ V. [Ti(H20).]4+ Select one: i, ii, and ili i, iii, v ii and iv iii and v A solution of 0.001 mol dm-3 NiSO4 is placed in an optical cell of pathlength 1 cm, and the absorption spectrum is recorded. The absorptions have characteristic Imax and Emax values. What are the correct units of Emax? Select one: mol dm-3 cm-1 cm mol dm-3 dm mol-1 cm-1 cm dm' mol-1 Choose the incorrect statement; Select one: O The Laporte rule implies that all dad transitions are forbidden O The Spin Rule states that transitions that involve the promotion of electrons without a change in their spin are allowed ; AS = 0 The Laporte rule states that there must be a change in parity, so Only g-->u or u-->g transitions are allowed The Laporte rule implies that all d-->d transitions are allowed. Calculate the energy Ao associated with 1/1 = 60 cm2, given that; . C = 3x10 m/s, 40 = E = hu h = 6.62x10-34 J.s Select one: 4x10 -22 J 12x10 -22 J 8x10-22 J 6x10 -22 J Choose the incorrect statement; Select one: O Dissociative mechanism takes place usually in 6-coordinate Oh complexes. O The rate determining step in Associative mechanism involves an intermediate formed with a higher C.N O Associative mechanism involves an intermediate which can be isolated and identified Associative mechanism plays a role in many d tetrahedral complexes Which ordering correctly describes the tendency of a ligand to direct ligand substitution in a square planar complex to a position trans to itself? Select one: CN > PR3 >1 > H20 CN> I > H2O > PR3 PR3> CN-> H20 > 1 I > CN > H2O > PR3 Choose the correct statement; Select one I is a good nucleophile in ligand substitution reactions and a good leaving group H2O is a good nuoleophile in ligand substitution reactions and a good leaving group CO is a good nucleophile in ligand substitution reactions and a good leaving group Interchange mechanism is a 2nd order substitution reaction which takes place in sq.planar complexes 1. Which of the following species would have the highest nucleophilicity parameter npt Select one: I- PhSH Ph3P SCN- Suppose you have the coordination compound : [Rh(en)3 ](ClO4)3, then the oxidation state of Rh, the d-electron count, and the magnetic properties for this complex would be : Select one: + 6d3 paramagnetic +3 db diamagnetic 0, d7 paramagnetic +3, do paramagnetic Choose the correct statement, The reaction profile below represents ; X.MY X-M.Y X.M-Y Reaction coordinate Select one: an elementary reaction Dissociative 2nd order mechanism of a complex reaction Dissociative 1st order mechanism of a complex reaction. Associative 2nd order mechanism of a complex reaction Clear my choice The reactions of (PtCl4]2- with PR3 followed by NH3 (reaction I) and of [PtCl4]2- with NH3 (reaction II) are ways of preparing: Select one: II: I: trans-[PtCl2(NH3)(PR3)}; trans-[PtCl (NH3)2] II: I: trans-[PtCl2(NH3)(PR3)];--II: cis-[PtCl2(NH3)2] I: cis-[PtCl2(NH3)(PR3)] cis-[PtCl2(NH3)2] I: cis-[PtCl2(NH3)(PR3)] II: trans-[PtCl2(NH3)2] Which of the following statements is incorrect? Select one: The electronic spectrum of [Ni(NH3).]2+ contains 3 absorptions Absorptions in the electronic spectrum of [Mn(OH2)6]2+ are extremely weak solutions of (Zn(OH2)6]2+ are expected to be colorless. The absorption in the electronic spectrum of (TI(OH2)6]3+ is assigned to the tag - eg transition - Which combination is correct for the complex ion in [Co(NH3)4(H20)Cl]Br? Oxidation Overall Shape of the state of charge of the cobalt complex ion complex ion a- +2 Oh +2 b- +3 Oh C- +2 Td d +2 Oh +1 Select one: a b d Choose the correct statement Select one: An elementary step has 0 intermediates & O transition states An elementary step has 0 intermediates & 1 transition state a complex reaction involves 1 intermediate & 1 transition state. An elementary step has 1 intermediate & 1 transition state Which ordering correctly describes the tendency of a ligand to direct ligand substitution in a square planar complex to a position opposite to itself? Select one: PR3> CO > NH3 > Br CO > Br > NH3 >PR3 CO >PR3 > Br"> NH3 Br > CO > NH3 >PR3 Choose the correct statement; Metal - PR3 p- bonding takes place between; Select one: a filled metal t2g d-orbital & an empty d- orbital of P atom a filled metal tag d-orbital & an empty p-orbital of P atom a filled metal eg d-orbital & an empty p-orbital of P atom a filled metal eg d-orbital & an empty d- orbital of P atom Which of the following absorption maxima is not in the visible range of the electronic spectrum? Select one: Imax = 830 nm max Imax = 430 nm Imax = 670 nm Imax = 530 nm Which statement is incorrect about [TI(OH2)613+? Select one: It is octahedral Solutions of the ion are coloured It is paramagnetic Considering only the d-orbitals, crystal field theory is consistent with a ground state electronic configuration of egt2g. Which of the following complexes would you expect to be colorless? i. [Y(H20)6]3+ ii: [Cr(en)313+ iii.(Cd(NH3)4]2+ iv. [CO(NH3)612+ V. [Ti(H20).]4+ Select one: i, ii, and ili i, iii, v ii and iv iii and v A solution of 0.001 mol dm-3 NiSO4 is placed in an optical cell of pathlength 1 cm, and the absorption spectrum is recorded. The absorptions have characteristic Imax and Emax values. What are the correct units of Emax? Select one: mol dm-3 cm-1 cm mol dm-3 dm mol-1 cm-1 cm dm' mol-1 Choose the incorrect statement; Select one: O The Laporte rule implies that all dad transitions are forbidden O The Spin Rule states that transitions that involve the promotion of electrons without a change in their spin are allowed ; AS = 0 The Laporte rule states that there must be a change in parity, so Only g-->u or u-->g transitions are allowed The Laporte rule implies that all d-->d transitions are allowed. Calculate the energy Ao associated with 1/1 = 60 cm2, given that; . C = 3x10 m/s, 40 = E = hu h = 6.62x10-34 J.s Select one: 4x10 -22 J 12x10 -22 J 8x10-22 J 6x10 -22 J Choose the incorrect statement; Select one: O Dissociative mechanism takes place usually in 6-coordinate Oh complexes. O The rate determining step in Associative mechanism involves an intermediate formed with a higher C.N O Associative mechanism involves an intermediate which can be isolated and identified Associative mechanism plays a role in many d tetrahedral complexes Which ordering correctly describes the tendency of a ligand to direct ligand substitution in a square planar complex to a position trans to itself? Select one: CN > PR3 >1 > H20 CN> I > H2O > PR3 PR3> CN-> H20 > 1 I > CN > H2O > PR3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


