Question: choose whether each one is positive, negative, or unknown Use the observations about each chemical reaction in the table below to decide the sign (positive
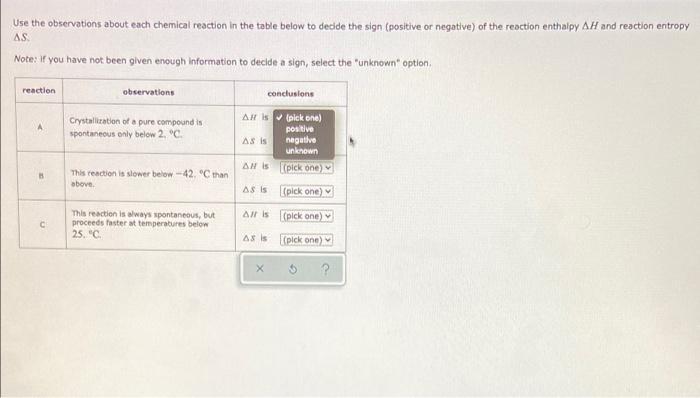
Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS Note: If you have not been given enough information to decide a sign, select the "unknown" option reaction observations conclusions A Crystallization of a pure compound is spontaneous only below 2.C AH (pick one) positive AS IS negative unknown pick one) This reaction is slower below-42 C than above, AS IS plek one) Alls (pick one) c This reaction is always spontaneous, but proceeds faster at temperatures below 25." ASI (pick one)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


