Question: Circle answer and show all work complete with proper number of significant figures for full credit (1) Calculate the root-mean-square velocity for the N2 molecules
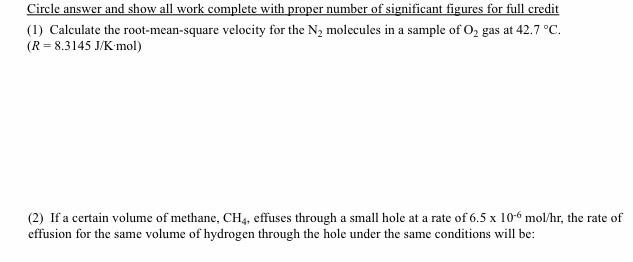
Circle answer and show all work complete with proper number of significant figures for full credit (1) Calculate the root-mean-square velocity for the N2 molecules in a sample of O2 gas at 42.7C. (R=8.3145J/Kmol) (2) If a certain volume of methane, CH4, effuses through a small hole at a rate of 6.5106mol/hr, the rate of effusion for the same volume of hydrogen through the hole under the same conditions will be
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


