Question: Classify each chemical reaction as a combination, precipitation, single replacement, combustion, double replacement, acid-base, decomposition. Classify each chemical reaction: reaction FeSO (aq) + PbCl (aq)
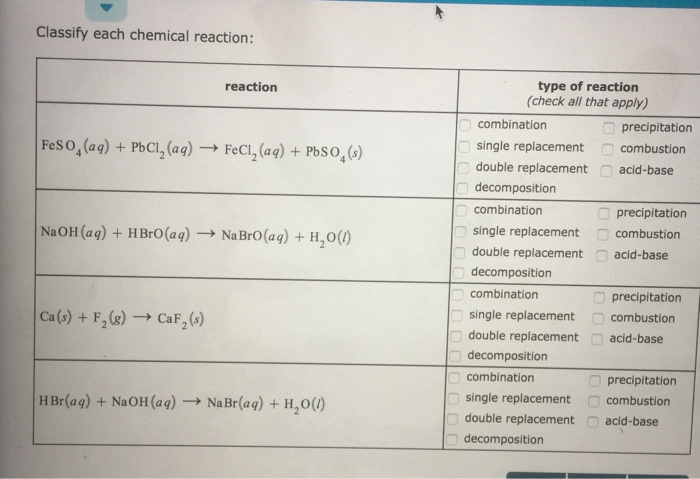
Classify each chemical reaction: reaction FeSO (aq) + PbCl (aq) FeCl (aq) + PbSO4(s) NaOH(aq) + HBrO (aq) Na BrO (aq) + HO(l) Ca (s) + F (g) CaF (s) HBr(aq) + NaOH(aq) NaBr(aq) + H,O(0) 00 type of reaction (check all that apply) combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition 00 000 precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


