Question: Classify each titration curve as representing a strong acid titrated with a strong base, a strong base titrated with a strong acid, a weak
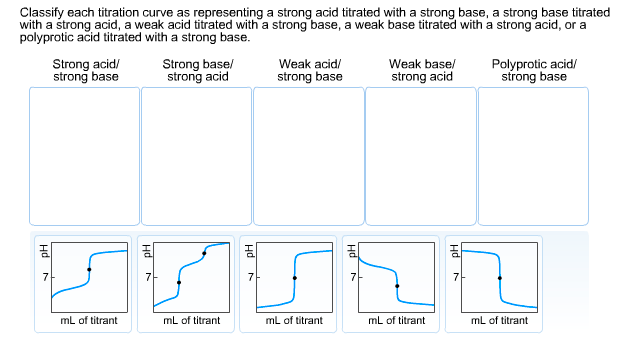
Classify each titration curve as representing a strong acid titrated with a strong base, a strong base titrated with a strong acid, a weak acid titrated with a strong base, a weak base titrated with a strong acid, or a polyprotic acid titrated with a strong base. Strong acid/ strong base Strong base/ strong acid mL of titrant Weak acid/ strong base mL of titrant FYSAL mL of titrant Weak base/ strong acid 7 mL of titrant Polyprotic acid/ strong base 7 mL of titrant
Step by Step Solution
3.48 Rating (161 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


