Question: Classroom test: Batch process engineering (CG5052) Nucleation experiments were performed in a research laboratory using glass vials of volume, V=20 mL, to study the nucleation
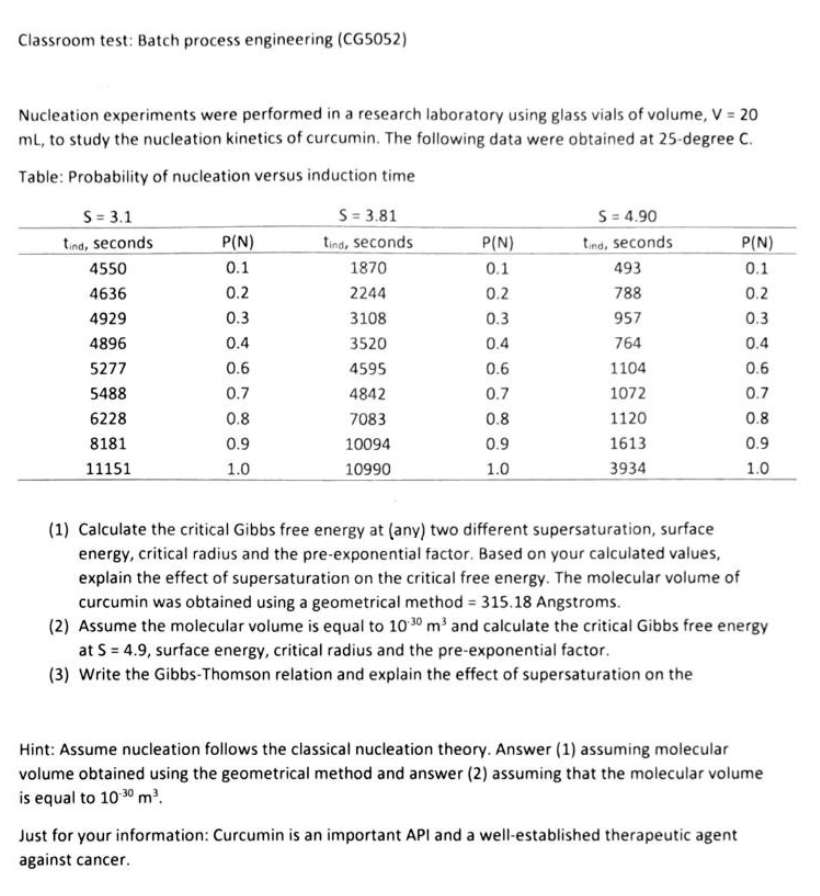
Classroom test: Batch process engineering (CG5052) Nucleation experiments were performed in a research laboratory using glass vials of volume, V=20 mL, to study the nucleation kinetics of curcumin. The following data were obtained at 25 -degree C. Table: Probability of nucleation versus induction time (1) Calculate the critical Gibbs free energy at (any) two different supersaturation, surface energy, critical radius and the pre-exponential factor. Based on your calculated values, explain the effect of supersaturation on the critical free energy. The molecular volume of curcumin was obtained using a geometrical method =315.18 Angstroms. (2) Assume the molecular volume is equal to 1030m3 and calculate the critical Gibbs free energy at S=4.9, surface energy, critical radius and the pre-exponential factor. (3) Write the Gibbs-Thomson relation and explain the effect of supersaturation on the Hint: Assume nucleation follows the classical nucleation theory. Answer (1) assuming molecular volume obtained using the geometrical method and answer (2) assuming that the molecular volume is equal to 1030m3. Just for your information: Curcumin is an important API and a well-established therapeutic agent against cancer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


