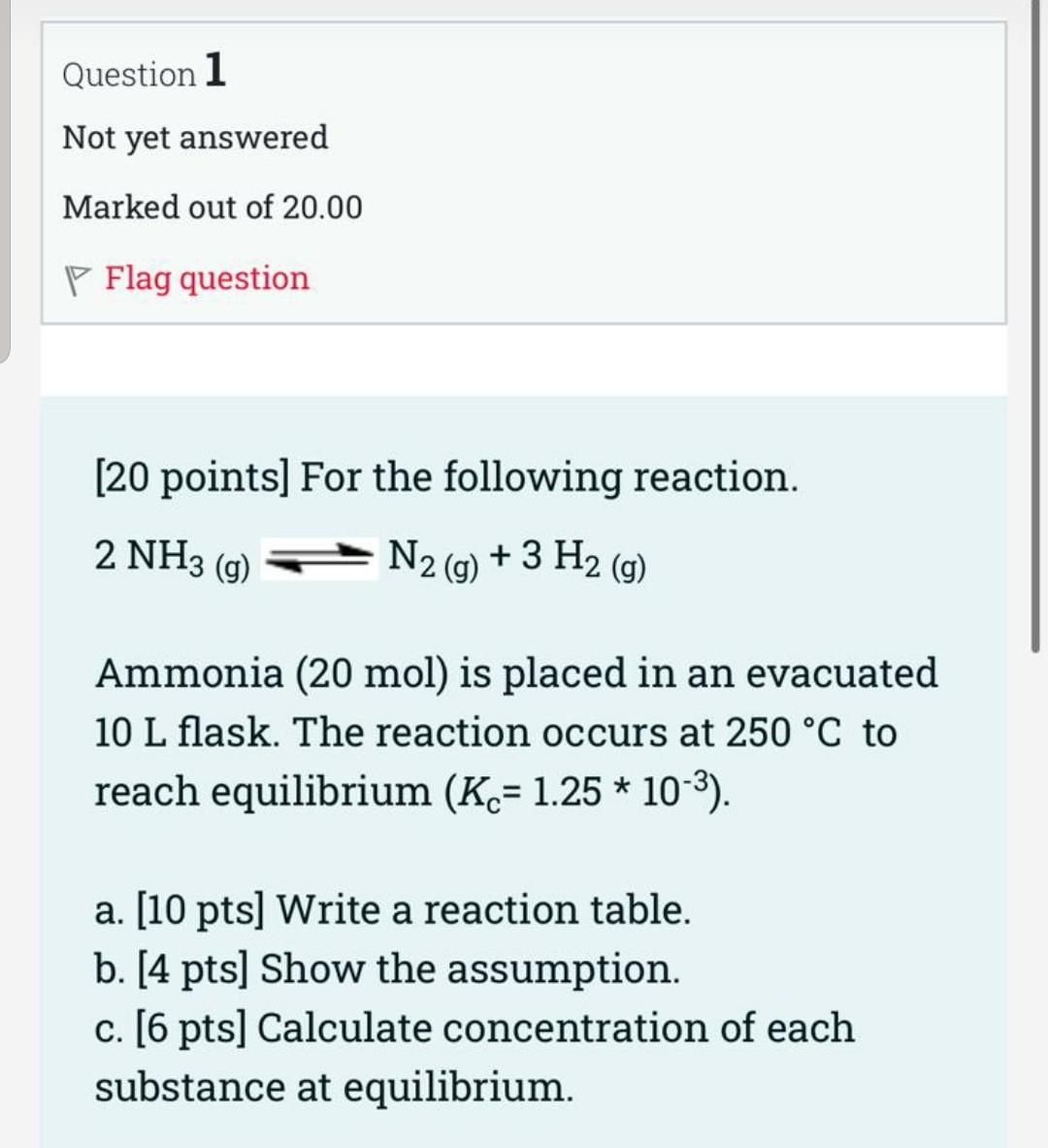
Question: clear handwritten please sir and correct answer Question 1 Not yet answered Marked out of 20.00 Flag question [20 points) For the following reaction. 2
clear handwritten please sir and correct answer
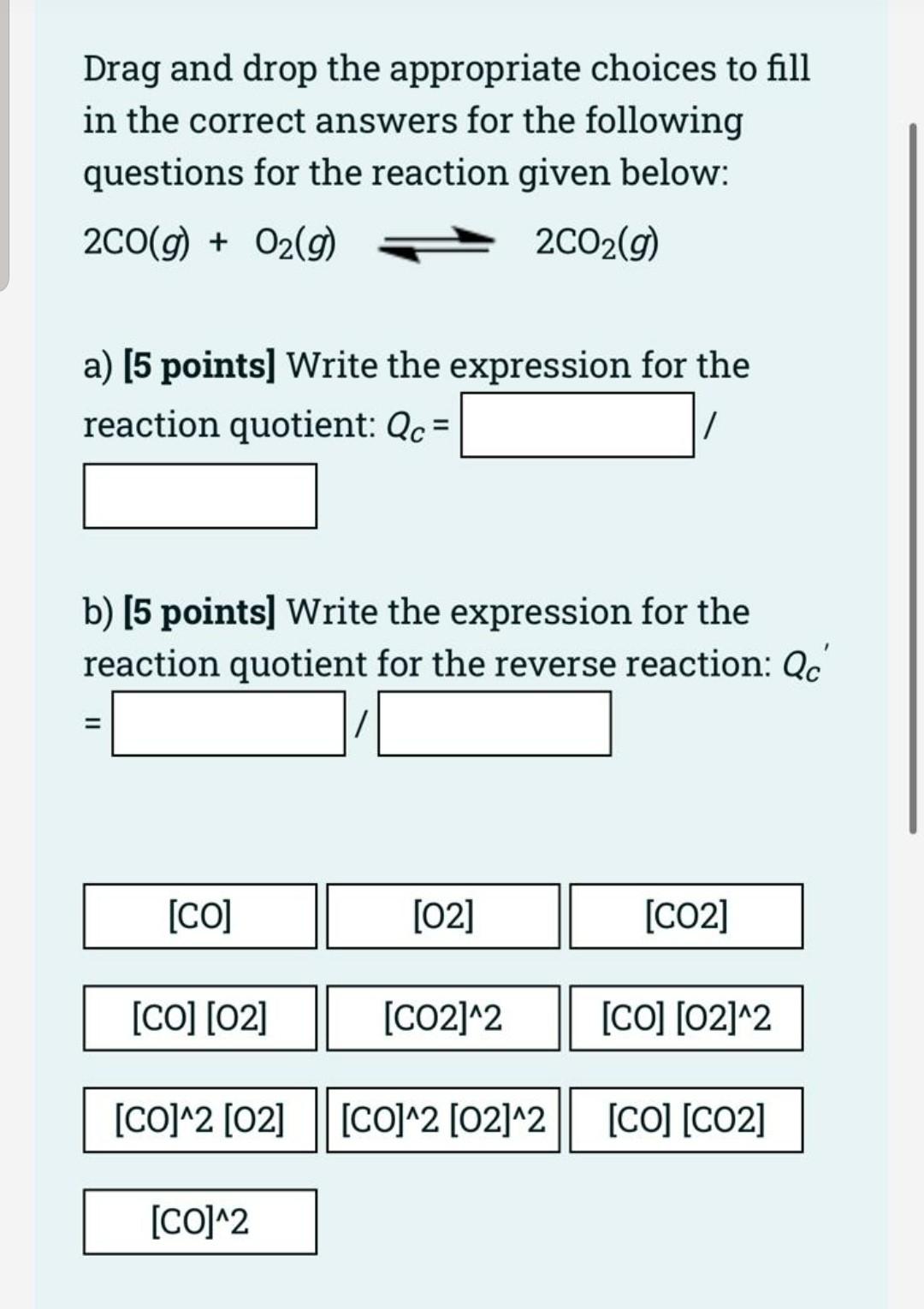
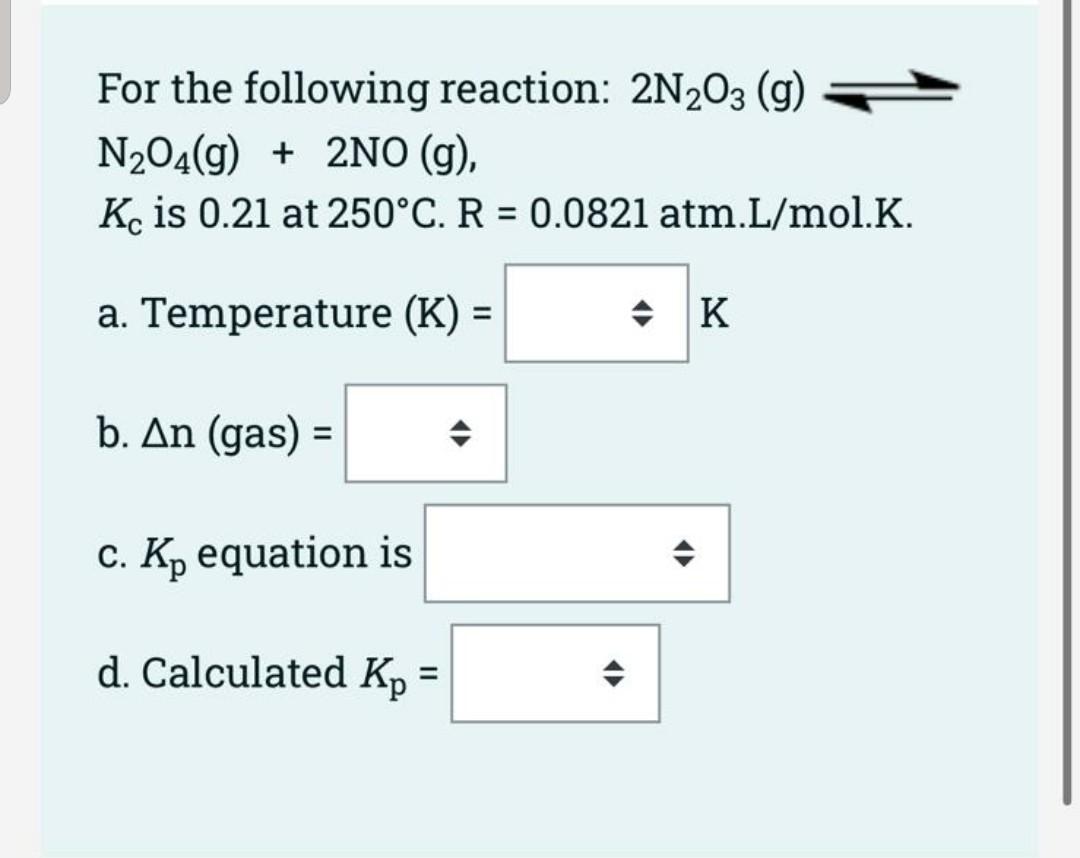
Question 1 Not yet answered Marked out of 20.00 Flag question [20 points) For the following reaction. 2 NH3(g) N2 (g) + 3 H2 (g) Ammonia (20 mol) is placed in an evacuated 10 L flask. The reaction occurs at 250 C to reach equilibrium (Kc= 1.25 * 10-3). * a. [10 pts) Write a reaction table. b. [4 pts] Show the assumption. c. [6 pts] Calculate concentration of each substance at equilibrium. Drag and drop the appropriate choices to fill in the correct answers for the following questions for the reaction given below: 2CO(g) + O2(g) 2C02(g) a) [5 points) Write the expression for the reaction quotient: Qc = / b) (5 points) Write the expression for the reaction quotient for the reverse reaction: Qc = [CO] [02] [CO2] [CO] [02] [CO2]^2 [CO] [02]^2 [CO]^2 [02] || [CO]^2 [02]^2 || [CO] [CO2] [CO]^2 For the following reaction: 2N203 (g) N204(g) + 2NO (g), K is 0.21 at 250C. R = 0.0821 atm.L/mol.K. = a. Temperature (K) = = * K b. An (gas) = c. Kp equation is d. Calculated Kp =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


