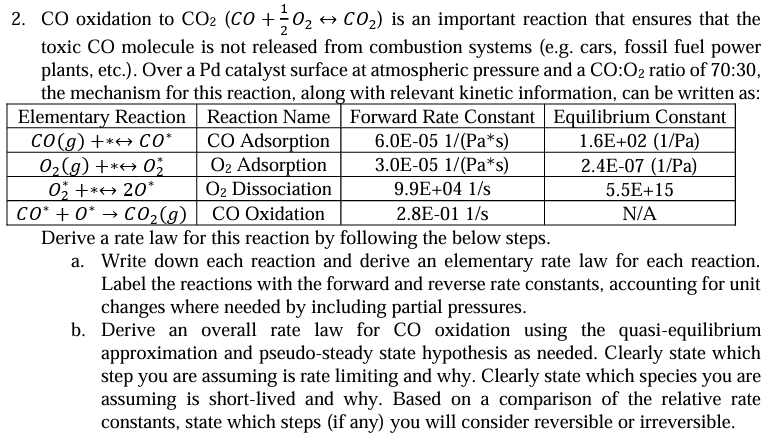
Question: CO oxidation to C O 2 ( C O + 1 2 O 2 h a r r C O 2 ) is an important
CO oxidation to is an important reaction that ensures that the
toxic molecule is not released from combustion systems eg cars, fossil fuel power
plants, etc. Over a Pd catalyst surface at atmospheric pressure and a : ratio of :
the mechanism for this reaction, along with relevant kinetic information, can be written as:
Derive a rate law for this reaction by following the below steps.
a Write down each reaction and derive an elementary rate law for each reaction.
Label the reactions with the forward and reverse rate constants, accounting for unit
changes where needed by including partial pressures.
b Derive an overall rate law for oxidation using the quasiequilibrium
approximation and pseudosteady state hypothesis as needed. Clearly state which
step you are assuming is rate limiting and why. Clearly state which species you are
assuming is shortlived and why. Based on a comparison of the relative rate
constants, state which steps if any you will consider reversible or irreversible.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


