Question: Col-0 O ASy is expected to be negative (a decrease in entropy) because two molecules are converted into one molecule. O Sy is expected to
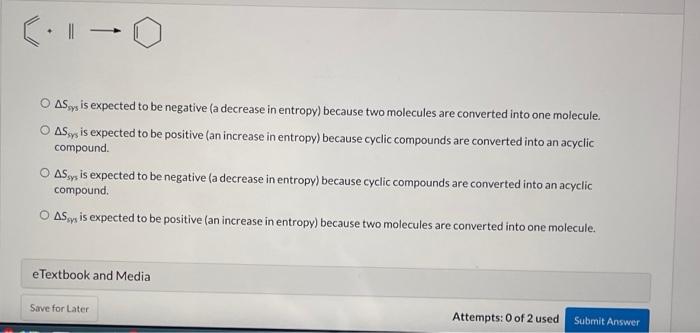
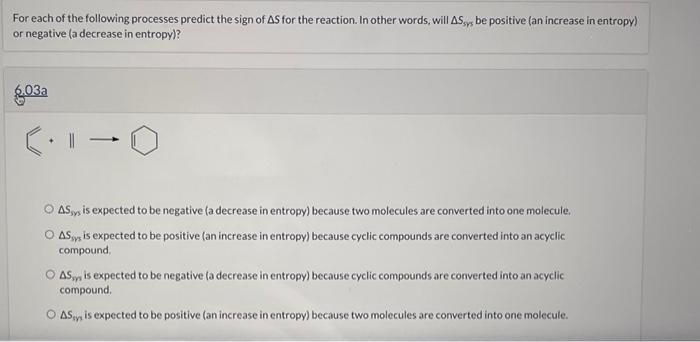
Col-0 O ASy is expected to be negative (a decrease in entropy) because two molecules are converted into one molecule. O Sy is expected to be positive (an increase in entropy) because cyclic compounds are converted into an acyclic compound O AS ,, is expected to be negative (a decrease in entropy) because cyclic compounds are converted into an acyclic compound Asoy is expected to be positive (an increase in entropy) because two molecules are converted into one molecule. e Textbook and Media Save for Later Attempts: 0 of 2 used Submit Answer For each of the following processes predict the sign of As for the reaction. In other words, will Asyys be positive (an increase in entropy) or negative (a decrease in entropy)? 803a 1 O ASy is expected to be negative (a decrease in entropy) because two molecules are converted into one molecule. O ASy is expected to be positive (an increase in entropy) because cyclic compounds are converted into an acyclic compound O AS is expected to be negative la decrease in entropy) because cyclic compounds are converted into an acyclic compound O AS is expected to be positive (an increase in entropy) because two molecules are converted into one molecule
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


