Question: Colligative constants can be found in the chempendix. Tf= Tb= Coligative Constants Constants for freezing-point depression and boiling-point elevation calculations at 1 atm: When using
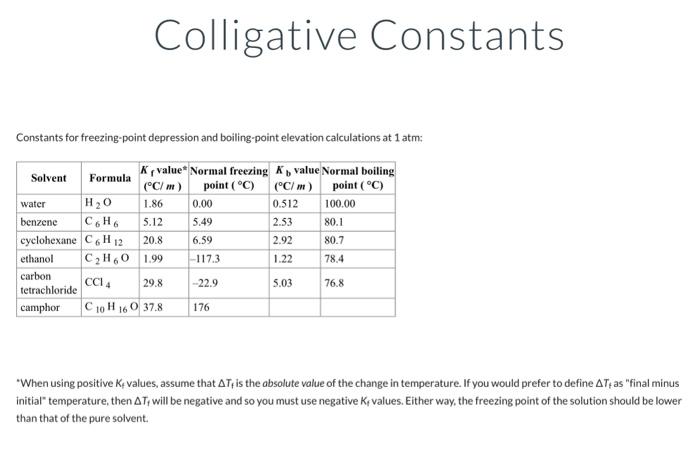
Colligative constants can be found in the chempendix. Tf= Tb= Coligative Constants Constants for freezing-point depression and boiling-point elevation calculations at 1 atm: "When using positive Kf values, assume that Tf is the absolute value of the change in temperature. If you would prefer to define Tf as "final minus initial" temperature, then Tf will be negative and so you must use negative Kf values. Either way, the freezing point of the solution should be lower than that of the pure solvent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


