Question: Complete Tables 4 & 5 using the equilibrium concentrations provided in the lab recording (Part 11). Table 4. Effect of temperature change on the equilibrium:
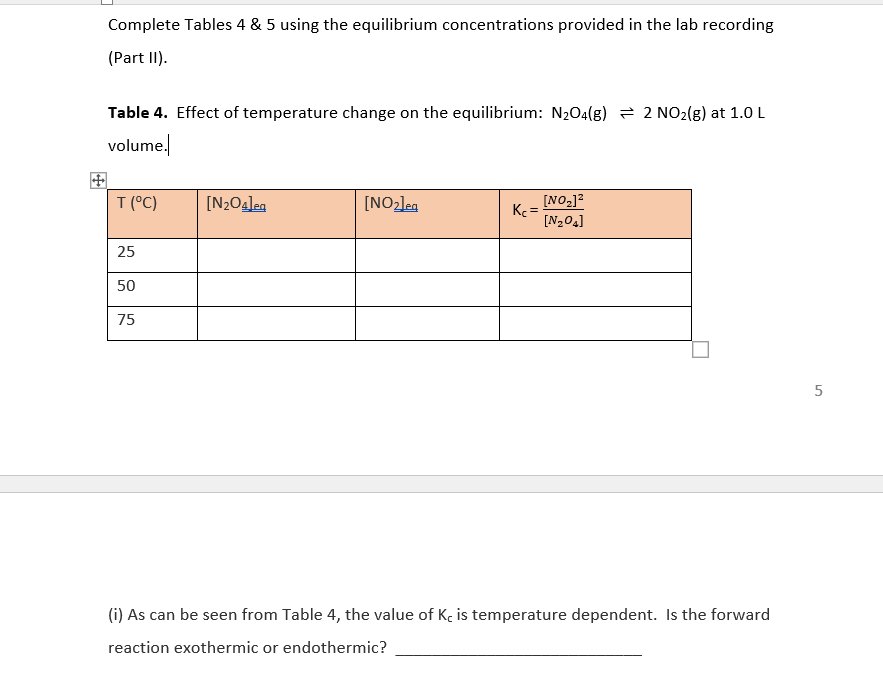
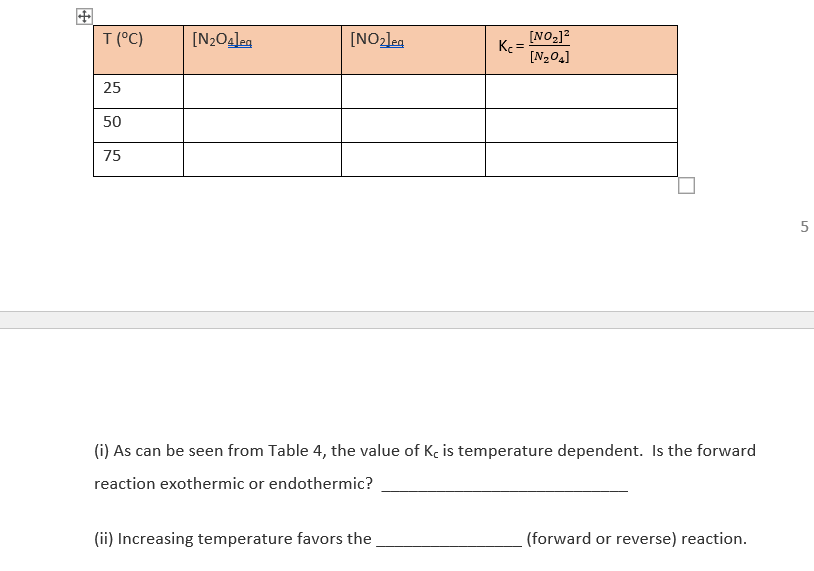
Complete Tables 4 & 5 using the equilibrium concentrations provided in the lab recording (Part 11). Table 4. Effect of temperature change on the equilibrium: N204(8) = 2 NO2(g) at 1.0 L volume. T(C) [N2O4]eg [NO2le [NO2)2 Ke= [N204] 25 50 75 5 (i) As can be seen from Table 4, the value of Kc is temperature dependent. Is the forward reaction exothermic or endothermic? T(C) [N2O42 [NO2leg [NO2]2 Ke= [N,04] 25 50 75 5 (i) As can be seen from Table 4, the value of Kc is temperature dependent. Is the forward reaction exothermic or endothermic? (ii) Increasing temperature favors the (forward or reverse) reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


