Question: complete the chart and solve the last three questions Table of Reagents and Product (Include units in all quantitative values, and use appropriate number of
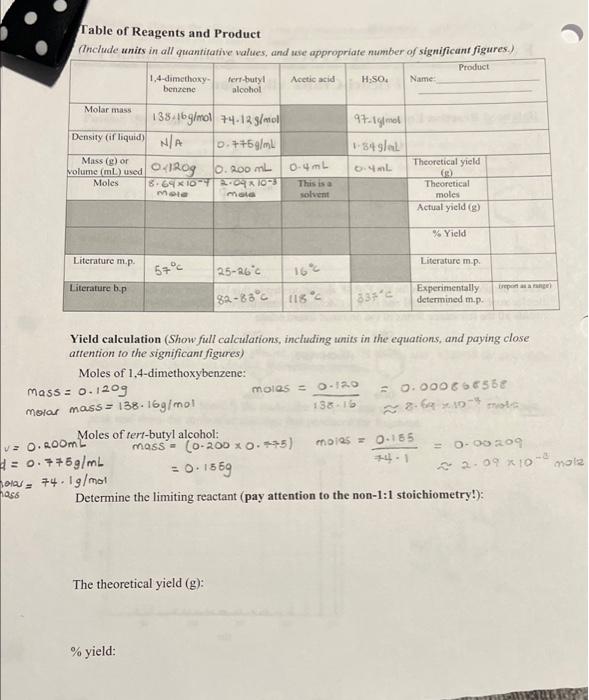
Table of Reagents and Product (Include units in all quantitative values, and use appropriate number of significant figures.) Product Acetic acid 1,4-dimethoxy bT4g Name ferr-butyl alcohol H SO Molar mass 97-1 mol 34 L 135.16g/mol 74.125/mol Density (if liquid) NA 0.7+5g/ml Mass (g) or wolume (ml) used 0 41R09 0.4mL Moles 8.69XIDT 2010- Mola b. 200 mL 0.4mL This is a Theoretical yield (9) Theoretical moles Actual yield (8) mau solvent % Yield Literature m.p. Literature m.p 55C Literature bp 25-26c 82-88C 118 pon 3 Experimentally determined m.p. Yield calculation (Show full calculations, including units in the equations, and paying close attention to the significant figures) Moles of 1,4-dimethoxybenzene: mass = 0.1209 mol = 0.12 = 0.0006350 morar mass = 138. 16g/mol 13 & 16 2.6 XDmoto Moles of tert-butyl alcohol: mass = (0-200 0.35) moins = 0.155 = 0.7+59/mL = 0.00209 = 0.1569 2-99X hol, 74.19/mol Determine the limiting reactant (pay attention to the non-1:1 stoichiometry!): V2.0. ROOM mal nass The theoretical yield (g): % yield: Table of Reagents and Product (Include units in all quantitative values, and use appropriate number of significant figures.) Product Acetic acid 1,4-dimethoxy bT4g Name ferr-butyl alcohol H SO Molar mass 97-1 mol 34 L 135.16g/mol 74.125/mol Density (if liquid) NA 0.7+5g/ml Mass (g) or wolume (ml) used 0 41R09 0.4mL Moles 8.69XIDT 2010- Mola b. 200 mL 0.4mL This is a Theoretical yield (9) Theoretical moles Actual yield (8) mau solvent % Yield Literature m.p. Literature m.p 55C Literature bp 25-26c 82-88C 118 pon 3 Experimentally determined m.p. Yield calculation (Show full calculations, including units in the equations, and paying close attention to the significant figures) Moles of 1,4-dimethoxybenzene: mass = 0.1209 mol = 0.12 = 0.0006350 morar mass = 138. 16g/mol 13 & 16 2.6 XDmoto Moles of tert-butyl alcohol: mass = (0-200 0.35) moins = 0.155 = 0.7+59/mL = 0.00209 = 0.1569 2-99X hol, 74.19/mol Determine the limiting reactant (pay attention to the non-1:1 stoichiometry!): V2.0. ROOM mal nass The theoretical yield (g): % yield
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


