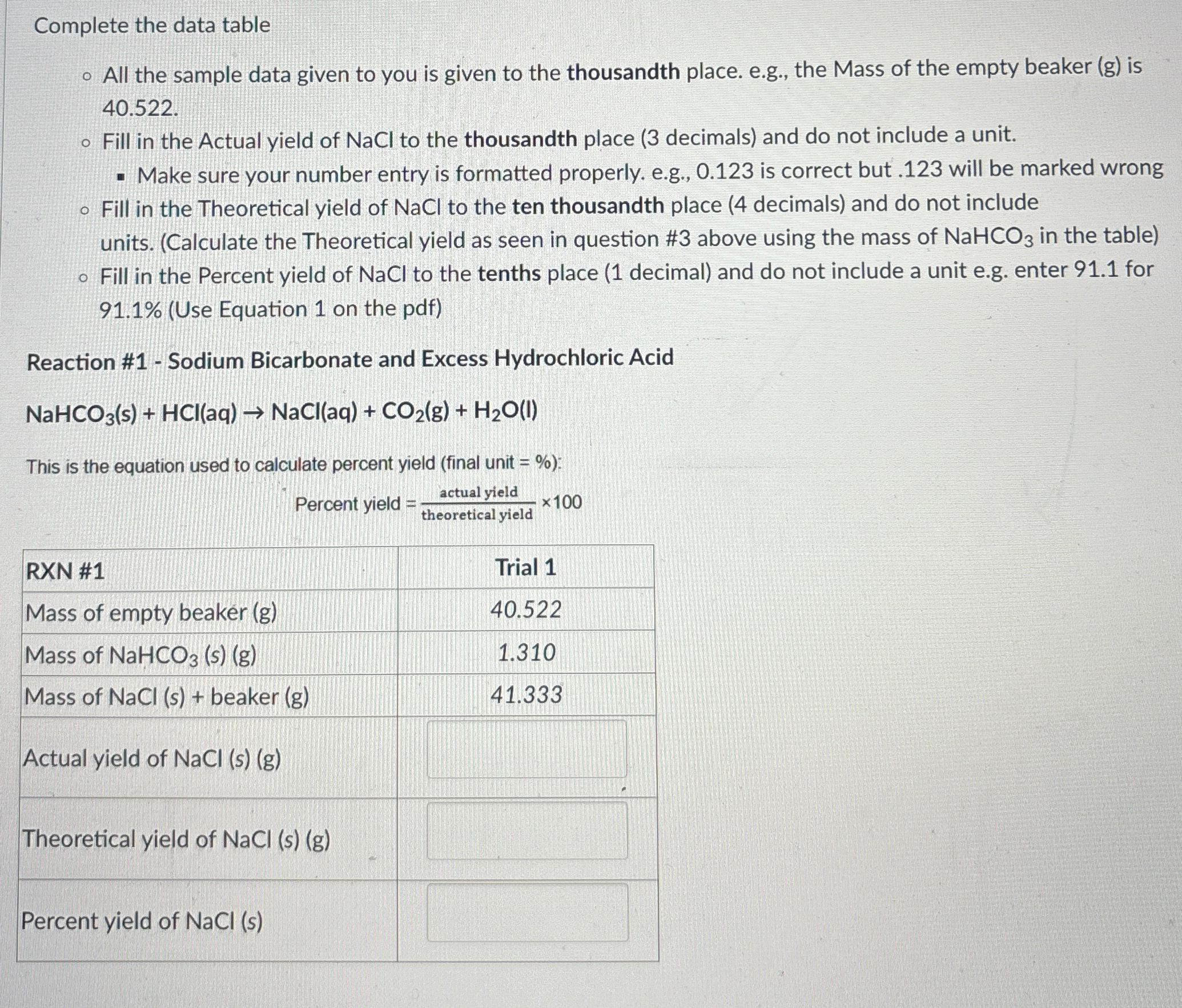
Question: Complete the data table All the sample data given to you is given to the thousandth place. e . g . , the Mass of
Complete the data table
All the sample data given to you is given to the thousandth place. eg the Mass of the empty beaker g is
Fill in the Actual yield of NaCl to the thousandth place decimals and do not include a unit.
Make sure your number entry is formatted properly. eg is correct but will be marked wrong
Fill in the Theoretical yield of NaCl to the ten thousandth place decimals and do not include units. Calculate the Theoretical yield as seen in question # above using the mass of in the table
Fill in the Percent yield of NaCl to the tenths place decimal and do not include a unit eg enter for Use Equation on the pdf
Reaction # Sodium Bicarbonate and Excess Hydrochloric Acid
NaCl
This is the equation used to calculate percent yield final unit :
Percent yield
tableRXN #Trial Mass of empty beaker Mass of :Mass of NaCl beaker Actual yield of NaClTheoretical yield of NaClPercent yield of NaCl
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


