Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [HO]....
Fantastic news! We've Found the answer you've been seeking!
Question:
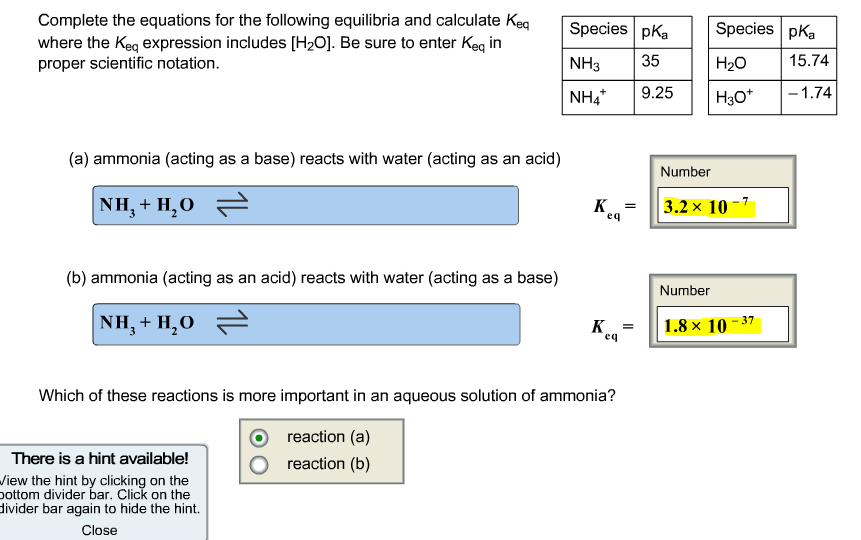
Transcribed Image Text:
Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [H₂O]. Be sure to enter Keq in proper scientific notation. (a) ammonia (acting as a base) reacts with water (acting as an acid) NH,+H,O = (b) ammonia (acting as an acid) reacts with water (acting as a base) NH,+H,O = Species pKa 35 9.25 There is a hint available! View the hint by clicking on the bottom divider bar. Click on the divider bar again to hide the hint. Close NH3 NH4* K eq K eq Which of these reactions is more important in an aqueous solution of ammonia? reaction (a) reaction (b) = Species pKa H₂O H3O+ Number 3.2x 10-7 Number 1.8 × 10-37 15.74 -1.74 Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [H₂O]. Be sure to enter Keq in proper scientific notation. (a) ammonia (acting as a base) reacts with water (acting as an acid) NH,+H,O = (b) ammonia (acting as an acid) reacts with water (acting as a base) NH,+H,O = Species pKa 35 9.25 There is a hint available! View the hint by clicking on the bottom divider bar. Click on the divider bar again to hide the hint. Close NH3 NH4* K eq K eq Which of these reactions is more important in an aqueous solution of ammonia? reaction (a) reaction (b) = Species pKa H₂O H3O+ Number 3.2x 10-7 Number 1.8 × 10-37 15.74 -1.74
Expert Answer:

Related Book For 

Posted Date:
Students also viewed these chemistry questions
-
Complete and balance the equations for the following reactions. a. Li(s) + N2(g) b. Rb(s) + S(s) c. Cs(s) + H2O(l) d. Na(s) + Cl2(g)
-
RECORD JOURNAL ENTRIES FOR THE TRANSACTIONS. BE SURE TO ENTER THE TRANSACTIONS IN PROPER JOURNAL FORM; THIS MEANS THAT THEY MUST BE NUMBERED AND DATED, AND INCLUDE A DESCRIPTION. 1) 1/2/2020 ISSUED...
-
Complete the equations for the ionization of an Arrhenius acid or base in water. Include the states of the products. HI (aq) ----------------> ________? LiOH (s) -----------------> ________?
-
A gaseous mixture consists of 80.0 mole percent N2 and 20.0 mole percent O2 (the approximate composition of air). Suppose water is saturated with the gas mixture at 25C and 1.00 atm total pressure,...
-
The General Electric Company has had its financial ups and downs. Recently, the CFO for General Electric helped turn its problems around by analyzing the amount of value each product was providing to...
-
Explain how Whole Foods decision will change the deadweight loss created by plastic straws. Whole foods to become first national supermarket to ban plastic straws Whole Foods is set to completely ban...
-
(3) From a stakeholder/influence perspective, what advantages do Nokia and Microsoft gain by collaborating?
-
Cook Corporation manufactures plastic garbage cans. In a typical year, the firm produces between 40,000 and 50,000 cans. At this level of production, fixed costs are $10,000 and variable costs are $2...
-
The Board of Directors of Teton Pearl, Inc., a private foundation, consists of Charlyne, Beth, and Carlos. They vote unanimously to provide a $931,000 grant to Carlos. The grant is to be used for...
-
a. Ash decides to allocate $4 million to fund the exhibit. Given the pieces available and the specific requirements from Ash and Celeste, formulate and solve a binary integer programming problem to...
-
Help me understand it Write the value of A, B, C, and D to one decimal place.Y \\oimavD: refer to some or all these numbers later In this assignment A: 50 B: 60 Q1. Consider Q: = -B HQ! Q2: -B HQ,...
-
The air in an automobile tire with a volume of \(0.015 \mathrm{~m}^{3}\) is at \(30^{\circ} \mathrm{C}\) and \(140 \mathrm{kPa}\) (gage). Determine the amount of air that must be added to raise the...
-
Convex Productions has just received a contract to film a commercial video that will air during a major sporting event in North America, and then be available on-demand through banner advertisements...
-
The following data (and annotations) for March 2016 are for the work in process account of the first of Olympus Companys four departments used in manufacturing its nly product. Assuming that Olympus...
-
If relative volatility can be assumed constant over the change in concentration for each fraction, Eq. \((9-13)\) can be adapted to the collection of fractions from a simple binary batch...
-
(a) Design a PI controller for Problem 8.6-4(b). (b) Design a PD controller for Problem 8.6-4(c). (c) Use the results of parts (a) and (b) to repeat Problem 8.6-4(d). Problem 8.6-4(b) (c) (d) (b)...
-
Al Smith, who lives in territory 5, carries 10/20/5 compulsory liability insurance along with optional collision that has a $300 deductible. Al, who was at fault in an accident, caused $4,000 damage...
-
Repeat Exercise 16.6 using the t-test of the coefficient of correlation. Is this result identical to the one you produced in Exercise 16.6?
-
Lenny Corporation was authorized to issue 27,000 shares of common stock . Record the journal entry for each of the following independent situations, assuming Lenny issues 6,200 shares at $17 on July...
-
Ada Company, using the periodic inventory system, began the year with 150 units of product B in inventory with a unit cost of $35. The following additional purchases of the product were made: Apr. 1...
-
Bob North and Whitney Adam are partners with capital balances of $1,500 and $600, respectively. They share all profits and losses equally. From the following independent situations, journalize the...
-
22. How are internal service funds reported on government-wide financial statements?
-
21. Why does a government determine the net expenses or revenues for each of the functions within its statement of activities?
-
20. What is the difference in program revenues and general revenues? Why is that distinction important?

Study smarter with the SolutionInn App


