Question: Complete the following questions: NOTE: When inputting an answer in scientific notation. Replace 103 with e-3. For example 1.50103 should be input as 1.50e3 a.
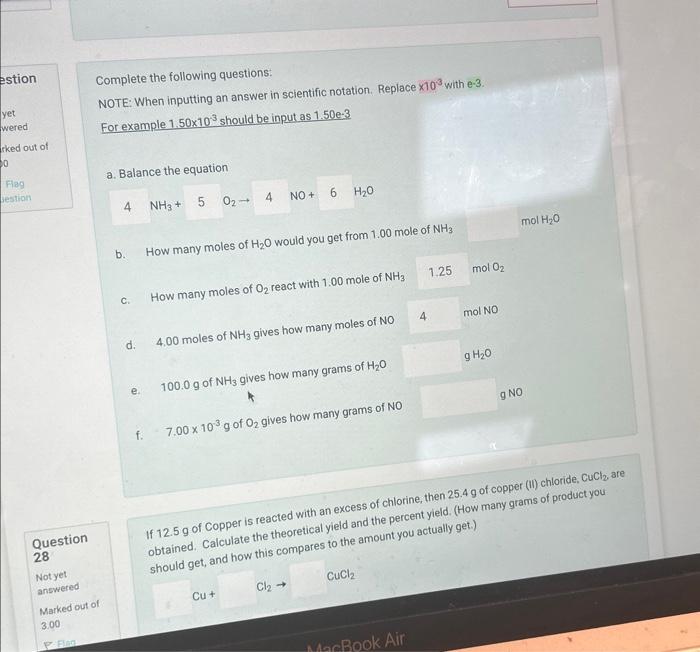
Complete the following questions: NOTE: When inputting an answer in scientific notation. Replace 103 with e-3. For example 1.50103 should be input as 1.50e3 a. Balance the equation 4NH3+5O24NO+6H2O b. How many moles of H2O would you get from 1.00 mole of NH3 d. 4,00 moles of NH3 gives how many moles of NO e. 100.0g of NH3 gives how many grams of H2O f. 7.00103g of O2 gives how many grams of NO Question 28 obtained. Calculate the theoretical yield and the percent yieid. (How Notyet answered Marked out of
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


