Question: Complete the following table. Table D. Calculated vs Measured pHs for Na,co3 Titration mL of 0.20 M HCI added Calculated pH Measured pH (from
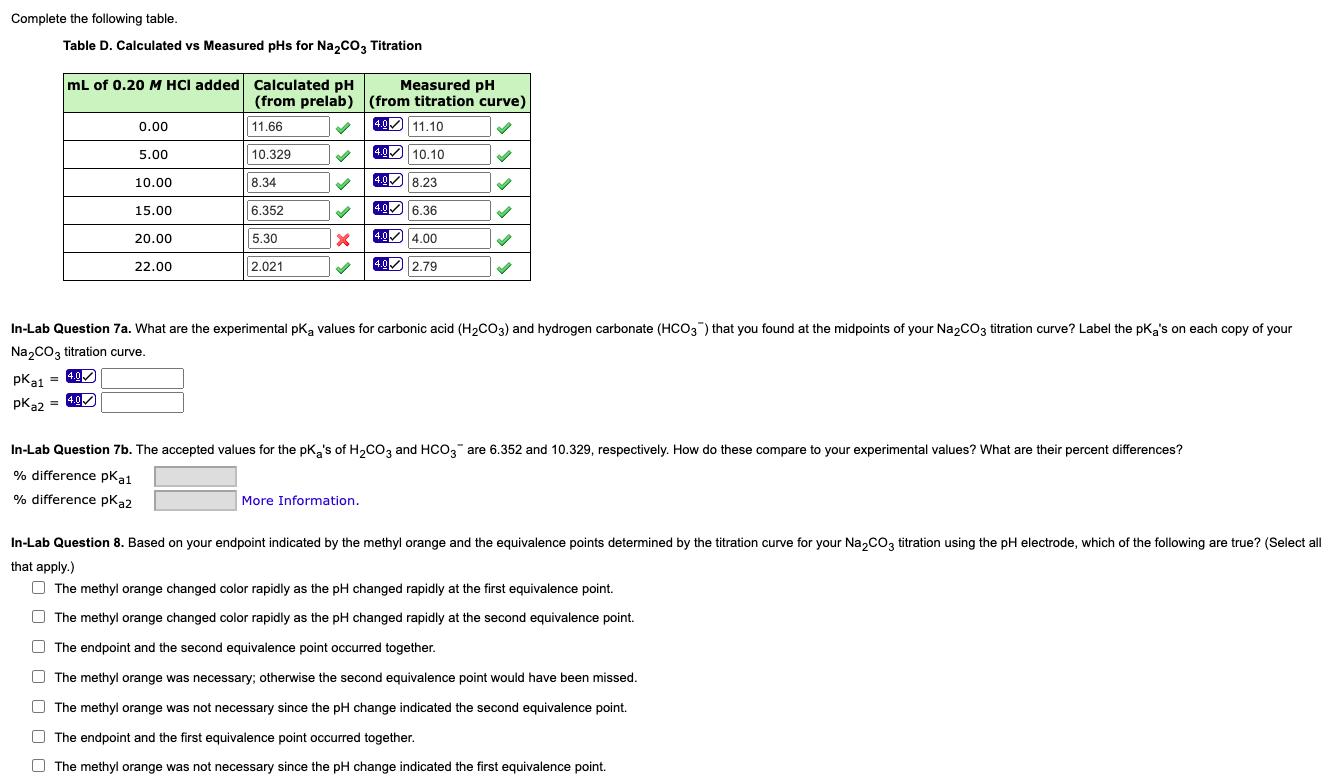
Complete the following table. Table D. Calculated vs Measured pHs for Na,co3 Titration mL of 0.20 M HCI added Calculated pH Measured pH (from prelab) (from titration curve) 49 11.10 0.00 11.66 4.0 5.00 10.329 4.0 10.10 8.34 40 8.23 10.00 40 6.36 15.00 6.352 5.30 494.00 20.00 2.021 40 2.79 22.00 In-Lab Question 7a. What are the experimental pk values for carbonic acid (H2CO3) and hydrogen carbonate (HCO3) that you found at the midpoints of your Na2CO3 titration curve? Label the pka's on each copy of your Na2CO3 titration curve. 4.0 pka1 = 40 pka2 = 4.0 In-Lab Question 7b. The accepted values for the pK,'s of H,Co, and HCO, are 6.352 and 10.329, respectively. How do these compare to your experimental values? What are their percent differences? % difference pKat % difference pka2 More Information. In-Lab Question 8. Based on your endpoint indicated by the methyl orange and the equivalence points determined by the titration curve for your Na,COz titration using the pH electrode, which of the following are true? (Select all that apply.) O The methyl orange changed color rapidly as the pH changed rapidly at the first equivalence point. O The methyl orange changed color rapidly as the pH changed rapidly at the second equivalence point. O The endpoint and the second equivalence point occurred together. O The methyl orange was necessary; otherwise the second equivalence point would have been missed. O The methyl orange was not necessary since the pH change indicated the second equivalence point. O The endpoint and the first equivalence point occurred together. O The methyl orange was not necessary since the pH change indicated the first equivalence point.
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it
InLab Question 7a pka1 40 pka2 49 InLab Question 7b The accepted values for ... View full answer

Get step-by-step solutions from verified subject matter experts


